Volume 9, Issue 4 (November 2022)
Avicenna J Neuro Psycho Physiology 2022, 9(4): 163-172 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Jamshidi Z, Karami M, Khalili M, Roghani M. Protective Effect of Baclofen against Oxidative Stress in Dorsal Hippocampus of Rats with Morphine-Induced Ovarian Polycystic. Avicenna J Neuro Psycho Physiology 2022; 9 (4) :163-172
URL: http://ajnpp.umsha.ac.ir/article-1-433-en.html
URL: http://ajnpp.umsha.ac.ir/article-1-433-en.html
1- Ph.D. Student, Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran
2- Associate Prof., Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran ,karami@shahed.ac.ir
3- Prof., Department of Physiology, Faculty of Medicine, Shahed University, Tehran, Iran
2- Associate Prof., Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran ,
3- Prof., Department of Physiology, Faculty of Medicine, Shahed University, Tehran, Iran
Full-Text [PDF 1756 kb]
(602 Downloads)
| Abstract (HTML) (2236 Views)
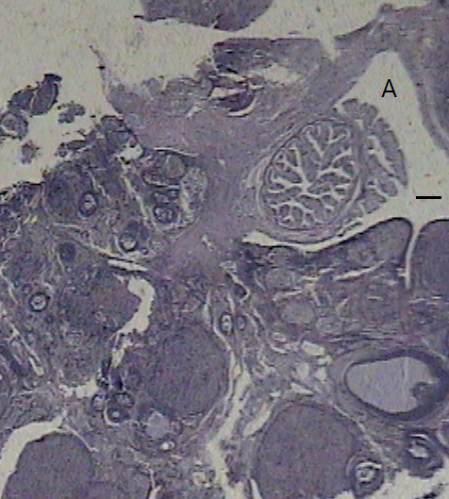




Figure 1. Ovary staining with hematoxylin-eosin. The ovary images belong to: A) control group, B) morphine (i.p.), C) morphine (intra-ventromedial hypothalamic), D) baclofen alone, and E) baclofen plus morphine. The line represents 50 µ.
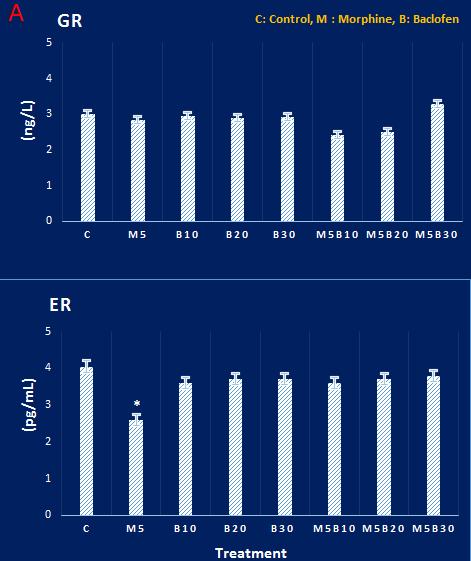
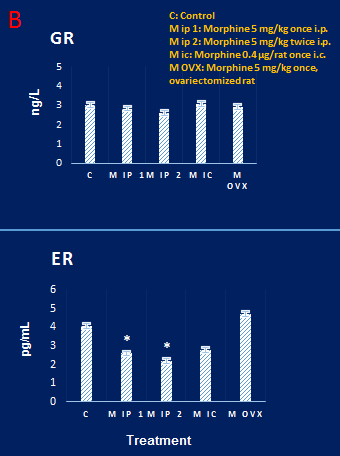
Figure 2. A and B. Concentrations of glucocorticoid and estrogen receptors in dorsal hippocampal tissue in different groups. Glucocorticoid receptor concentration did not show any significant differences among different groups. There were also no significant differences among the estrogen receptor concentration of the groups, except the morphine group, which showed a significant decrease, compared to the control group. P<0.05, based on Tukey’s post hoc.

Figure 3. Results of immunohistochemical staining of glucocorticoid receptors in rat hippocampus. The density of these receptors in the hippocampal tissue of the control animal (A) and other groups, such as the morphine group (B), showed no difference. The scale lines in A and B represent 10 µ and 50 µ, respectively
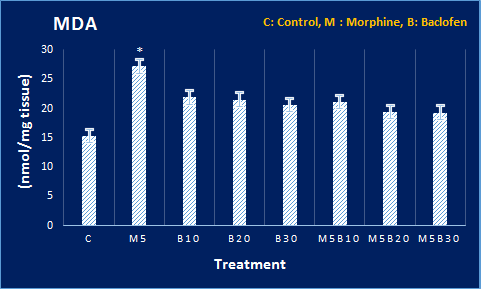
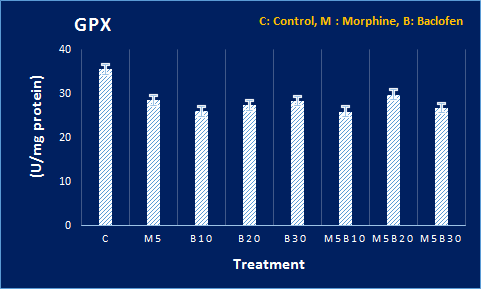
Figure 4. Levels of oxidative stress mediators at DH in all groups. The MDA and glutathione peroxidase levels and also activities of CAT and SOD enzymes in DH tissue were compared with the control group. The MDA level showed an increase, and SOD and CAT activities underwent a decrease, compared to the control group. P<0.05 signifies the difference, compared to the control group, based on Tukey’s post hoc.


Figure 5. Levels of serum testosterone in all groups. Its level showed a decrease in morphine groups, compared to the control group. P<0.05 shows the difference with control based on Tukey’s post hoc.
Full-Text: (982 Views)
Background
Ovarian cystogenesis is one of the main causes of infertility in women [1]. It is associated with abnormal secretion of gonadotropins, increased production of male sex steroids in the ovaries, hirsutism, insulin-dependent hypersensitivity, and infertility [2,3]. The pathophysiology of this phenomenon is complex and its basis has not yet been determined clearly, but some causes are listed as reproductive axis and ovarian dysfunctions [1].
In ovarian cystogenesis, the inhibitory feedback effect of steroid hormones on Gonadotropin-releasing hormone (GnRH) neurons is disrupted, resulting in increased GnRH secretion that produces luteinizing hormone (LH). The increased steady and rapid pulse rate of LH hormone in adults can cause hyperandrogenism and ovulation disorder [4,5]. In women with polycystic ovary syndrome (PCOS), testosterone and LH levels increase and follicle-stimulating hormone (FSH) and estrogen levels decrease [6]. The prevalence rate of this disorder among women of reproductive age has been reported to be ≥ 6% [7,8]. It should be noted that a part of this percentage relates to those who use morphine (and narcotics) in large quantities [9-11].
Morphine, with the chemical formula C17H19NO3, is the most important alkaloid found in opium extract [12,13]. It is widely prescribed for the treatment of pain as it produces an analgesic effect by modulating pre- and post-synaptic gamma-aminobutyric acid (GABA) neurotransmission associated with adenylyl cyclase [14]. However, narcotics, such as morphine, especially in case of long-term use, have many side effects, including polycystic ovary (PCO), which disrupts the hypothalamus-pituitary-gonadal axis [15,16].
According to the statistics released by the United Nations, more than 200 million people worldwide are addicted to drugs. The mean age of the onset of drug abuse in women is 15 years which includes their reproductive age range [17]. Morphine targets the reproductive system through mu-opioid receptors (MORs). After stimulation of MORs, which are dependent on Gi protein, adenylate cyclase and voltage-gated Ca2+ channels are inhibited and K+ channels are activated, which leads to a decrease in reproductive axis activity [18]. The MOR receptors also cause the release of nitric oxide (NO) from the postsynaptic terminal, which triggers inflammatory pathways [19].
In addition, morphine has adverse effects on the central nervous system, including neurotoxicity, neurological disorders, oxidative stress, and apoptosis [20]. One of the most affected areas of the brain is the hippocampal regions [21], as during stress, glucocorticoid hormones secreted by the adrenal cortex reach the hippocampus through the bloodstream and activate glucocorticoid receptors at the hippocampus. They increase the calcium uptake into CA1 cells, which causes oxidative stress [22].
The resulting neuronal damage can be inhibited by potent GABA receptor agonists [23]. The GABA has been shown to have protective effects against metabolic disorders and PCOS [24,25]. Baclofen is a specific and selective GABAB receptor agonist, which is a phenolic derivative of GABA (gamma-aminobutyric acid) and has an analgesic effect. It crosses the blood-brain barrier (opposed to GABA) [26,27]. Until now, baclofen has shown a protective effect against the inflammatory effects caused by morphine consumption [23].
The hypothesis of PCO-induced oxidative stress damage in CA1 has not been investigated before. Therefore, the present study aimed to investigate the protective effect of baclofen as a strong GABA receptor agonist on oxidative stress caused by PCO associated with morphine in the rat hippocampus. It is hoped that this research can be a helpful step toward the solution of the side effects induced by morphine.
Objectives
This study aimed to investigate the protective effect of baclofen against oxidative stress in the dorsal hippocampus (DH) of morphine-treated rats with ovarian cysts.
Materials and Methods
Animal Subject and Grouping
In this experimental study, 66 female virgin rats (Wistar) within the weight range of 220-250 g were purchased from Pasteur Institute in Tehran, Iran. They were kept in animal Laboratory standard conditions with a 12-h light/dark cycle at 21-25 °C with free access to water and food ad libitum in accordance with the declaration of Helsinki. It must be mentioned that the Ethics Committee of Shahed University, Tehran, Iran approved the study (IR.
SHAHED.REC.1399.114).
The female rats were divided into 11 groups (6 rats in each group): the control group (placebo) received saline (1 mL/kg) , once a day intraperitoneally (i.p.), two groups received morphine (5 mg/kg) once and twice i.p., once daily, six groups were administered baclofen (10, 20, and 30 mg/kg, once a day alone or as pre-injection relative to morphine, one group received morphine (0.4 µg/rat) once as an intra-ventromedial hypothalamic (VMH) nucleus injection, and the last gonadectomized group was administered morphine (5 mg kg) once inside the peritoneum after a week recovery.
Used Drugs
The drugs used in this research included morphine sulfate (Tolid Mavade Avallieh Daroupakhsh or Temad Co., Tehran, Iran) and baclofen (Temad Co., Tehran, Iran). These substances were provided with the official permission of the Ministry of Health. Moreover, ketamine (10% or 100 mg/kg) and xylazine (2% or 20 mg/kg) were provided by the Veterinary Organization of Iran.
Blood Sampling and Serology
The rats were anesthetized with ketamine-xylazine 24 h after the treatments. In the next step, blood samples were obtained from their hearts under anesthesia. After blood collection, the samples were placed in the laboratory for about 1 h to let the blood coagulate. The test tubes were then centrifuged at 3000 rpm for 10 min. Serums were isolated and biochemically examined for LH, FSH, testosterone, and blood lipid profile levels (to investigate the role of obesity as a potential cause of PCO).
Histopathology
With a longitudinal incision in the abdominal surface of rats (under anesthesia), their ovaries and uterus were exposed from the abdominal cavity. After the removal of the surrounding adipose tissue, their dimensions were measured using a caliper, and then these tissues were placed in formalin. The samples were fixed for 48-72 h; afterward, slices with a thickness of 3-4 μ were prepared by a microtome device (Leica, Germany). Finally, ovaries and uterus were examined histologically for cysts and other parameters after staining with hematoxylin-eosin.
Ovariectomy
For ovariectomy, on the day of surgery, the animals were first anesthetized using ketamine (100 mg/kg) and xylazine (20 mg/kg). Subsequently, a vertical incision was made to the midline on the side of the animal from the tail to the last rib. An opening in the muscular wall was created on each side, the ovary was removed, and the surgical site was sutured with 5.0 silk thread. Afterward, the site was carefully cleaned with betadine (Povidone-iodine) and amoxicillin powder.
Intra-ventromedial hypothalamus injection
Once under anesthesia with ketamine (100 mg/kg) and xylazine (20 mg/kg) and under the control of the stereotaxis device (Stolting Co., U.S.A), the rats were cannulated in the VMH coordinates (see 16). One week later, when recovery was achieved, the animals were administered morphine (0.4 μg/rat, intra-VMH) using a Hamilton syringe. The control group in this experiment only received saline (1 μl/rat, intra-VMH).
Brains samples
Brains of the animals were removed; subsequently, the hippocampal regions were isolated on ice, placed in liquid nitrogen, and finally put in a freezer at -80 °C for future studies. To prepare brain slices, these samples were kept for 24 h in sucrose phosphate buffer (30%) at 4 °C and then cut with a cryostat (Leica, Germany). Cresyl violet staining was used to evaluate the condition of dorsal hippocampal (DH) neurons. This dye is specifically used to show Nissl bodies in the cytoplasm of neurons and is also commonly used to detect necrosis in the brain tissue.
After removal of the hippocampus specimen from the -80 °C freezer, it was dehydrated, cleared, embedded in paraffin, sliced (3-4 µ), and placed on slides coated with poly L-lysine. After two steps of paraffin removal in xylene, the sample was immersed in alcohol 100%, 95%, 80%, 70%, and 50%, washed with distilled water, put in cresyl violet (0.1 g in 100 mL of distilled water) for 30 min, and washed again with distilled water. After dewatering with ascending degrees of alcohol and clarification with xylene, it was mounted using Entellan (Merck, Germany) and studied under a photomicroscope (Olympus, US).
Density of estrogen and glucocorticoid receptors in dorsal hippocampus by enzyme-linked immune-sorbent assay method
For this purpose, estrogen receptor and cortisol receptor enzyme-linked immunosorbent assay (ELISA) kits (Zellbio Co., Germany) were used. First, 1 mL of phosphate buffer was added for every 100 mg of hippocampal tissue to create tissue homogeneity. Afterward, it was homogenized with a homogenizer and then centrifuged. To prevent protein degradation, 1 μL of antiprotease (phenylmethylsulfonyl fluoride) was added for every 1 mL of the prepared solution. After centrifugation, the supernatant was separated and used for the assay. The sample was rechecked and recoded by ELISA reader at 450 nm. Serial dilutions and standard curves were also prepared separately, and the receptors concentrations were calculated by using the curve and its equation.
Examination of estrogen and glucocorticoid receptors in the dorsal hippocampus by immune-histochemistry
After two stages of tissue paraffinization in xylene (each for 30 min), the DH samples were placed in 100%, 96%, 90%, and 80% alcohols for 5 min in two stages. Afterward, they were put in distilled water for 5 min and then in citrate buffer (pH=7.4). Subsequently, hydrogen peroxide (incubation for 10 min), buffer (twice), and primary antibodies (ab16660 for estrogen receptor and ab183127 for cortisol receptor) were added.
Each slice was washed 4 times in the buffer; afterward, a sufficient amount of multi-capacity biotinylated solution was added to them. Subsequently, the sample was incubated for 10 min at room temperature and then washed 4 times in the buffer. Streptavidin peroxidase was then added, incubated at room temperature for 10 min, washed 4 times in the buffer, exposed to 3,3'-Diaminobenzidine chromogen (for 1-10 min), and finally washed 4 times in the buffer again.
Evaluation of oxidative stress factors (i.e., superoxide dismutase, catalase, glutathione peroxidase, and malondialdehyde) in the dorsal hippocampus
Glutathione peroxidase assay
In this method, a glutathione peroxidase (GPX) activity test kit (Zellbio, Germany with Cat. No: ZB-GPX-A96) was used. The enzyme glutathione peroxidase catalyzes the oxidation reaction of glutathione by cumene hydroperoxide. In the presence of the enzymes glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADP), the oxidized glutathione is converted back to regenerative glutathione, which is associated with the simultaneous oxidation of NADP to NADP +. In this reaction, the decrease in light absorption was measured at 412 nm. The obtained absorption value was multiplied by the amount of enzyme activity in international units per mg of tissue protein and then reported.
Malondialdehyde measurement:
Malondialdehyde (MDA) measurement was performed using the ZellBio kit (Cat. No: ZB-MDA96). Serial dilutions of standard solution were prepared and used to draw the standard curve. The MDA of the samples was calculated using this curve and its equation. Each sample was examined twice and read at 535 nm.
Assay of superoxide dismutase enzyme:
A test of enzyme activity was performed using a superoxide dismutase assay (ZellBio Cat. No: ZB-SOD-96A). This kit uses a reaction value to convert superoxide anion to hydrogen peroxide and oxygen. The unit of superoxide dismutase activity represents the conversion of superoxide anions to oxygen and hydrogen peroxide.
Catalase Enzyme Assay:
Measurement of catalase (CAT) enzyme activity was performed using the catalase activity assay kit (Zellbio Cat No: ZB-CAT-96A). Serial dilutions of standard solution were prepared and used to draw the standard curve. The amount of catalase in the samples was calculated using this curve and its equation. Each sample was prepared twice and read at 405 nm in the ELISA reader.
Study of activation of pro-inflammatory nitric oxide system in the ovary and dorsal hippocampus by histochemical nicotinamide adenine dinucleotide phosphate-diaphorase.
To show the activity of the NO synthase enzyme in the ovary and DH, after preparing tissue sections with a thickness of 4 µ, these sections were placed on the poly-L-lysine slides. Subsequently, they were clarified in two stages with xylene, diluted in 50-100% alcohol, and exposed to equal proportions of NADP-d (2 mg per 1 mL of phosphate buffer) and nitro blue tetrazolium chloride (0.4 mg per 1 mL of phosphate buffer). The samples were then incubated at 37 °C for 16 h, rinsed with tap water, dehydrated in 50-100% alcohol, clarified in xylene, glued with Entellan (Merck, Germany), and examined under a microscope.
Statistical analysis
Results were evaluated by one-way analysis of variance and when the difference was significant, they were computed by Tukey's Post hoc test with α=0.05. Image Software (version 2023) was used to quantitatively examine the images and also see the baclofen-morphine and morphine challenge in one graph and compare the different procedures in another graph.
Results
Number of ovarian cysts in morphine and baclofen receiving groups
In the ovaries of the control group, follicles were observed at different stages of development. However, in the groups receiving morphine, regardless of the route of injection (i.p. or intra-VMH), the developing follicles were rare but the thick-walled follicular cysts were abundant. In the groups that received single baclofen or baclofen with morphine, the number of cysts was significantly reduced, while the number of mature follicles underwent an increase (Figure 1).
In ovarian cystogenesis, the inhibitory feedback effect of steroid hormones on Gonadotropin-releasing hormone (GnRH) neurons is disrupted, resulting in increased GnRH secretion that produces luteinizing hormone (LH). The increased steady and rapid pulse rate of LH hormone in adults can cause hyperandrogenism and ovulation disorder [4,5]. In women with polycystic ovary syndrome (PCOS), testosterone and LH levels increase and follicle-stimulating hormone (FSH) and estrogen levels decrease [6]. The prevalence rate of this disorder among women of reproductive age has been reported to be ≥ 6% [7,8]. It should be noted that a part of this percentage relates to those who use morphine (and narcotics) in large quantities [9-11].
Morphine, with the chemical formula C17H19NO3, is the most important alkaloid found in opium extract [12,13]. It is widely prescribed for the treatment of pain as it produces an analgesic effect by modulating pre- and post-synaptic gamma-aminobutyric acid (GABA) neurotransmission associated with adenylyl cyclase [14]. However, narcotics, such as morphine, especially in case of long-term use, have many side effects, including polycystic ovary (PCO), which disrupts the hypothalamus-pituitary-gonadal axis [15,16].
According to the statistics released by the United Nations, more than 200 million people worldwide are addicted to drugs. The mean age of the onset of drug abuse in women is 15 years which includes their reproductive age range [17]. Morphine targets the reproductive system through mu-opioid receptors (MORs). After stimulation of MORs, which are dependent on Gi protein, adenylate cyclase and voltage-gated Ca2+ channels are inhibited and K+ channels are activated, which leads to a decrease in reproductive axis activity [18]. The MOR receptors also cause the release of nitric oxide (NO) from the postsynaptic terminal, which triggers inflammatory pathways [19].
In addition, morphine has adverse effects on the central nervous system, including neurotoxicity, neurological disorders, oxidative stress, and apoptosis [20]. One of the most affected areas of the brain is the hippocampal regions [21], as during stress, glucocorticoid hormones secreted by the adrenal cortex reach the hippocampus through the bloodstream and activate glucocorticoid receptors at the hippocampus. They increase the calcium uptake into CA1 cells, which causes oxidative stress [22].
The resulting neuronal damage can be inhibited by potent GABA receptor agonists [23]. The GABA has been shown to have protective effects against metabolic disorders and PCOS [24,25]. Baclofen is a specific and selective GABAB receptor agonist, which is a phenolic derivative of GABA (gamma-aminobutyric acid) and has an analgesic effect. It crosses the blood-brain barrier (opposed to GABA) [26,27]. Until now, baclofen has shown a protective effect against the inflammatory effects caused by morphine consumption [23].
The hypothesis of PCO-induced oxidative stress damage in CA1 has not been investigated before. Therefore, the present study aimed to investigate the protective effect of baclofen as a strong GABA receptor agonist on oxidative stress caused by PCO associated with morphine in the rat hippocampus. It is hoped that this research can be a helpful step toward the solution of the side effects induced by morphine.
Objectives
This study aimed to investigate the protective effect of baclofen against oxidative stress in the dorsal hippocampus (DH) of morphine-treated rats with ovarian cysts.
Materials and Methods
Animal Subject and Grouping
In this experimental study, 66 female virgin rats (Wistar) within the weight range of 220-250 g were purchased from Pasteur Institute in Tehran, Iran. They were kept in animal Laboratory standard conditions with a 12-h light/dark cycle at 21-25 °C with free access to water and food ad libitum in accordance with the declaration of Helsinki. It must be mentioned that the Ethics Committee of Shahed University, Tehran, Iran approved the study (IR.
SHAHED.REC.1399.114).
The female rats were divided into 11 groups (6 rats in each group): the control group (placebo) received saline (1 mL/kg) , once a day intraperitoneally (i.p.), two groups received morphine (5 mg/kg) once and twice i.p., once daily, six groups were administered baclofen (10, 20, and 30 mg/kg, once a day alone or as pre-injection relative to morphine, one group received morphine (0.4 µg/rat) once as an intra-ventromedial hypothalamic (VMH) nucleus injection, and the last gonadectomized group was administered morphine (5 mg kg) once inside the peritoneum after a week recovery.
Used Drugs
The drugs used in this research included morphine sulfate (Tolid Mavade Avallieh Daroupakhsh or Temad Co., Tehran, Iran) and baclofen (Temad Co., Tehran, Iran). These substances were provided with the official permission of the Ministry of Health. Moreover, ketamine (10% or 100 mg/kg) and xylazine (2% or 20 mg/kg) were provided by the Veterinary Organization of Iran.
Blood Sampling and Serology
The rats were anesthetized with ketamine-xylazine 24 h after the treatments. In the next step, blood samples were obtained from their hearts under anesthesia. After blood collection, the samples were placed in the laboratory for about 1 h to let the blood coagulate. The test tubes were then centrifuged at 3000 rpm for 10 min. Serums were isolated and biochemically examined for LH, FSH, testosterone, and blood lipid profile levels (to investigate the role of obesity as a potential cause of PCO).
Histopathology
With a longitudinal incision in the abdominal surface of rats (under anesthesia), their ovaries and uterus were exposed from the abdominal cavity. After the removal of the surrounding adipose tissue, their dimensions were measured using a caliper, and then these tissues were placed in formalin. The samples were fixed for 48-72 h; afterward, slices with a thickness of 3-4 μ were prepared by a microtome device (Leica, Germany). Finally, ovaries and uterus were examined histologically for cysts and other parameters after staining with hematoxylin-eosin.
Ovariectomy
For ovariectomy, on the day of surgery, the animals were first anesthetized using ketamine (100 mg/kg) and xylazine (20 mg/kg). Subsequently, a vertical incision was made to the midline on the side of the animal from the tail to the last rib. An opening in the muscular wall was created on each side, the ovary was removed, and the surgical site was sutured with 5.0 silk thread. Afterward, the site was carefully cleaned with betadine (Povidone-iodine) and amoxicillin powder.
Intra-ventromedial hypothalamus injection
Once under anesthesia with ketamine (100 mg/kg) and xylazine (20 mg/kg) and under the control of the stereotaxis device (Stolting Co., U.S.A), the rats were cannulated in the VMH coordinates (see 16). One week later, when recovery was achieved, the animals were administered morphine (0.4 μg/rat, intra-VMH) using a Hamilton syringe. The control group in this experiment only received saline (1 μl/rat, intra-VMH).
Brains samples
Brains of the animals were removed; subsequently, the hippocampal regions were isolated on ice, placed in liquid nitrogen, and finally put in a freezer at -80 °C for future studies. To prepare brain slices, these samples were kept for 24 h in sucrose phosphate buffer (30%) at 4 °C and then cut with a cryostat (Leica, Germany). Cresyl violet staining was used to evaluate the condition of dorsal hippocampal (DH) neurons. This dye is specifically used to show Nissl bodies in the cytoplasm of neurons and is also commonly used to detect necrosis in the brain tissue.
After removal of the hippocampus specimen from the -80 °C freezer, it was dehydrated, cleared, embedded in paraffin, sliced (3-4 µ), and placed on slides coated with poly L-lysine. After two steps of paraffin removal in xylene, the sample was immersed in alcohol 100%, 95%, 80%, 70%, and 50%, washed with distilled water, put in cresyl violet (0.1 g in 100 mL of distilled water) for 30 min, and washed again with distilled water. After dewatering with ascending degrees of alcohol and clarification with xylene, it was mounted using Entellan (Merck, Germany) and studied under a photomicroscope (Olympus, US).
Density of estrogen and glucocorticoid receptors in dorsal hippocampus by enzyme-linked immune-sorbent assay method
For this purpose, estrogen receptor and cortisol receptor enzyme-linked immunosorbent assay (ELISA) kits (Zellbio Co., Germany) were used. First, 1 mL of phosphate buffer was added for every 100 mg of hippocampal tissue to create tissue homogeneity. Afterward, it was homogenized with a homogenizer and then centrifuged. To prevent protein degradation, 1 μL of antiprotease (phenylmethylsulfonyl fluoride) was added for every 1 mL of the prepared solution. After centrifugation, the supernatant was separated and used for the assay. The sample was rechecked and recoded by ELISA reader at 450 nm. Serial dilutions and standard curves were also prepared separately, and the receptors concentrations were calculated by using the curve and its equation.
Examination of estrogen and glucocorticoid receptors in the dorsal hippocampus by immune-histochemistry
After two stages of tissue paraffinization in xylene (each for 30 min), the DH samples were placed in 100%, 96%, 90%, and 80% alcohols for 5 min in two stages. Afterward, they were put in distilled water for 5 min and then in citrate buffer (pH=7.4). Subsequently, hydrogen peroxide (incubation for 10 min), buffer (twice), and primary antibodies (ab16660 for estrogen receptor and ab183127 for cortisol receptor) were added.
Each slice was washed 4 times in the buffer; afterward, a sufficient amount of multi-capacity biotinylated solution was added to them. Subsequently, the sample was incubated for 10 min at room temperature and then washed 4 times in the buffer. Streptavidin peroxidase was then added, incubated at room temperature for 10 min, washed 4 times in the buffer, exposed to 3,3'-Diaminobenzidine chromogen (for 1-10 min), and finally washed 4 times in the buffer again.
Evaluation of oxidative stress factors (i.e., superoxide dismutase, catalase, glutathione peroxidase, and malondialdehyde) in the dorsal hippocampus
Glutathione peroxidase assay
In this method, a glutathione peroxidase (GPX) activity test kit (Zellbio, Germany with Cat. No: ZB-GPX-A96) was used. The enzyme glutathione peroxidase catalyzes the oxidation reaction of glutathione by cumene hydroperoxide. In the presence of the enzymes glutathione reductase and nicotinamide adenine dinucleotide phosphate (NADP), the oxidized glutathione is converted back to regenerative glutathione, which is associated with the simultaneous oxidation of NADP to NADP +. In this reaction, the decrease in light absorption was measured at 412 nm. The obtained absorption value was multiplied by the amount of enzyme activity in international units per mg of tissue protein and then reported.
Malondialdehyde measurement:
Malondialdehyde (MDA) measurement was performed using the ZellBio kit (Cat. No: ZB-MDA96). Serial dilutions of standard solution were prepared and used to draw the standard curve. The MDA of the samples was calculated using this curve and its equation. Each sample was examined twice and read at 535 nm.
Assay of superoxide dismutase enzyme:
A test of enzyme activity was performed using a superoxide dismutase assay (ZellBio Cat. No: ZB-SOD-96A). This kit uses a reaction value to convert superoxide anion to hydrogen peroxide and oxygen. The unit of superoxide dismutase activity represents the conversion of superoxide anions to oxygen and hydrogen peroxide.
Catalase Enzyme Assay:
Measurement of catalase (CAT) enzyme activity was performed using the catalase activity assay kit (Zellbio Cat No: ZB-CAT-96A). Serial dilutions of standard solution were prepared and used to draw the standard curve. The amount of catalase in the samples was calculated using this curve and its equation. Each sample was prepared twice and read at 405 nm in the ELISA reader.
Study of activation of pro-inflammatory nitric oxide system in the ovary and dorsal hippocampus by histochemical nicotinamide adenine dinucleotide phosphate-diaphorase.
To show the activity of the NO synthase enzyme in the ovary and DH, after preparing tissue sections with a thickness of 4 µ, these sections were placed on the poly-L-lysine slides. Subsequently, they were clarified in two stages with xylene, diluted in 50-100% alcohol, and exposed to equal proportions of NADP-d (2 mg per 1 mL of phosphate buffer) and nitro blue tetrazolium chloride (0.4 mg per 1 mL of phosphate buffer). The samples were then incubated at 37 °C for 16 h, rinsed with tap water, dehydrated in 50-100% alcohol, clarified in xylene, glued with Entellan (Merck, Germany), and examined under a microscope.
Statistical analysis
Results were evaluated by one-way analysis of variance and when the difference was significant, they were computed by Tukey's Post hoc test with α=0.05. Image Software (version 2023) was used to quantitatively examine the images and also see the baclofen-morphine and morphine challenge in one graph and compare the different procedures in another graph.
Results
Number of ovarian cysts in morphine and baclofen receiving groups
In the ovaries of the control group, follicles were observed at different stages of development. However, in the groups receiving morphine, regardless of the route of injection (i.p. or intra-VMH), the developing follicles were rare but the thick-walled follicular cysts were abundant. In the groups that received single baclofen or baclofen with morphine, the number of cysts was significantly reduced, while the number of mature follicles underwent an increase (Figure 1).





Figure 1. Ovary staining with hematoxylin-eosin. The ovary images belong to: A) control group, B) morphine (i.p.), C) morphine (intra-ventromedial hypothalamic), D) baclofen alone, and E) baclofen plus morphine. The line represents 50 µ.
Uterus tissue
The uterus did not show significant changes in any group.
Estrogen and glucocorticoid receptors intensities in the dorsal hippocampus
The enzyme-linked immunosorbent assay (ELISA) results showed that the concentration of glucocorticoid receptors in DH did not show
significant differences in different groups (Figure 2). However, the density of estrogen receptors in the morphine-receiving group showed a decrease, compared to the control group. Results of the immunohistochemical study revealed that the level of expression of these receptors in the control group showed no obvious difference, compared to the group treated with morphine (Figure 3).
The uterus did not show significant changes in any group.
Estrogen and glucocorticoid receptors intensities in the dorsal hippocampus
The enzyme-linked immunosorbent assay (ELISA) results showed that the concentration of glucocorticoid receptors in DH did not show
significant differences in different groups (Figure 2). However, the density of estrogen receptors in the morphine-receiving group showed a decrease, compared to the control group. Results of the immunohistochemical study revealed that the level of expression of these receptors in the control group showed no obvious difference, compared to the group treated with morphine (Figure 3).


Figure 2. A and B. Concentrations of glucocorticoid and estrogen receptors in dorsal hippocampal tissue in different groups. Glucocorticoid receptor concentration did not show any significant differences among different groups. There were also no significant differences among the estrogen receptor concentration of the groups, except the morphine group, which showed a significant decrease, compared to the control group. P<0.05, based on Tukey’s post hoc.


Figure 3. Results of immunohistochemical staining of glucocorticoid receptors in rat hippocampus. The density of these receptors in the hippocampal tissue of the control animal (A) and other groups, such as the morphine group (B), showed no difference. The scale lines in A and B represent 10 µ and 50 µ, respectively
Blood lipid profile
Triglycerides, total cholesterol, LDL, and HDL were tested. The results showed no significant differences between different groups in terms of blood lipid profile (not shown).
Nitric oxide involvement at the ovary, uterus, and dorsal hippocampus
The results showed that in the morphine-receiving groups, the NO system was not activated in ovarian and DH tissues (not shown).
Dorsal hippocampal neurons
Necrosis in the CA1 region did not develop in neurons in any of the studied groups (not shown).
Level of oxidative stress at dorsal hippocampus
Based on the results, the level of MDA in DH was significantly increased in the morphine groups, compared to the control group. Moreover, the level of MDA in DH increased significantly in the morphine-receiving groups, compared to the control group. However, there was less difference between baclofen-morphine and the control groups in this regard due to the protective effect of baclofen. In addition, the activity of CAT and superoxide dismutase (SOD) enzymes in the morphine-receiving groups showed a significant decrease, compared to the control group. Besides, the GPX level showed no significant change (Figure 4).
Triglycerides, total cholesterol, LDL, and HDL were tested. The results showed no significant differences between different groups in terms of blood lipid profile (not shown).
Nitric oxide involvement at the ovary, uterus, and dorsal hippocampus
The results showed that in the morphine-receiving groups, the NO system was not activated in ovarian and DH tissues (not shown).
Dorsal hippocampal neurons
Necrosis in the CA1 region did not develop in neurons in any of the studied groups (not shown).
Level of oxidative stress at dorsal hippocampus
Based on the results, the level of MDA in DH was significantly increased in the morphine groups, compared to the control group. Moreover, the level of MDA in DH increased significantly in the morphine-receiving groups, compared to the control group. However, there was less difference between baclofen-morphine and the control groups in this regard due to the protective effect of baclofen. In addition, the activity of CAT and superoxide dismutase (SOD) enzymes in the morphine-receiving groups showed a significant decrease, compared to the control group. Besides, the GPX level showed no significant change (Figure 4).
 |
 |
 |
 |
 |
 |
 |
 |
Serology
Levels of FSH, LH, and testosterone in sera samples were measured. According to the results, there was a significant decrease in the androgen hormone levels in morphine-treated groups, compared to the control group (Figure 5), while the levels of gonadotropin hormones were not changed.
Biometry of ovaries and uterus
The results did not show a significant difference among the studied groups (not shown).
Discussion
Abuse of drugs, including morphine, has become a worldwide issue nowadays. Morphine has adverse effects on the nervous system [28], and baclofen can interfere with the results of morphine use [29]. This study investigated the protective effects of baclofen dosages [30] on oxidative stress in the DH in an experimental model of acute morphine-induced ovarian cystogenesis.
Until now, various substances have been used to induce PCO (e.g., letrozole, testosterone, estradiol valerate, hydroepiandrosterone, adrenocorticotropin, and even long-term exposure light) [31]. Morphine has also been shown to induce ovarian cystogenesis by stimulating the pro-inflammatory nitric oxide (NO) system [32]. However, in this research, except for the marginal lining of the ovary, none of the parts of its main frame showed a positive reaction to the biochemical marker of NO. Now, it is not clear whether there is a problem with the method and a more suitable method should be used to show the activation and stimulation of this system. Currently, this is one of the limitations of this research, but it is suggested that future studies investigate this issue.
The mechanisms involved in PCO caused by morphine are also not clear yet. Studies have shown that hypothalamic-pituitary-adrenal axis activity increases in PCO [33]. Some researchers believe that hypothalamic-pituitary-adrenal and sympathetic activity trigger a stress response that causes physiological and metabolic changes in the body and that the stress and elevated glucocorticoids in the blood cause oxidative stress [34].
In the present study, the concentration of glucocorticoid receptors at DH was specifically examined. However, the results showed no significant changes in glucocorticoid receptor levels at DH, identifying a questionable involvement. Therefore, further investigation is required to find out if the brain is damaged by oxidative stress processes under these conditions (PCO). The hippocampus is highly sensitive to oxidative stress [22]; however, its sensitivity to oxidative stress in the morphine-induced PCO model has not been demonstrated yet. In the present study, the level of oxidative stress in DH was higher in the morphine-treated groups with ovarian cysts, compared to the control group. Hence, morphine probably plays a role in ovarian cystogenesis by oxidative stress in DH. Nevertheless, it is not clear whether morphine is the most effective component or its metabolites.
In mammals, morphine (i.p.) is mainly metabolized in the liver by the uridine-5-phospho-glucuronosyltransferase enzyme to two glucuronide metabolites (morphine-3-glucuronide and morphine-6-glucuronide). The M3G is produced five times more than M6G, but only M6G can cross the blood-brain barrier (BBB). Moreover, M6G has been shown to cross BBB 7.5 times less than morphine. It tends to bind to the opioid μ-receptor, but due to its lower levels than morphine, its analgesic activity is significantly lower in comparison to morphine [35].
In the present study, to compare the ef6
fectiveness of morphine and its metabolites in inducing oxidative stress in the brain, an effective dose of morphine was injected into the VMH [see 16 for dose selection]. However, no significant
Levels of FSH, LH, and testosterone in sera samples were measured. According to the results, there was a significant decrease in the androgen hormone levels in morphine-treated groups, compared to the control group (Figure 5), while the levels of gonadotropin hormones were not changed.
Biometry of ovaries and uterus
The results did not show a significant difference among the studied groups (not shown).
Discussion
Abuse of drugs, including morphine, has become a worldwide issue nowadays. Morphine has adverse effects on the nervous system [28], and baclofen can interfere with the results of morphine use [29]. This study investigated the protective effects of baclofen dosages [30] on oxidative stress in the DH in an experimental model of acute morphine-induced ovarian cystogenesis.
Until now, various substances have been used to induce PCO (e.g., letrozole, testosterone, estradiol valerate, hydroepiandrosterone, adrenocorticotropin, and even long-term exposure light) [31]. Morphine has also been shown to induce ovarian cystogenesis by stimulating the pro-inflammatory nitric oxide (NO) system [32]. However, in this research, except for the marginal lining of the ovary, none of the parts of its main frame showed a positive reaction to the biochemical marker of NO. Now, it is not clear whether there is a problem with the method and a more suitable method should be used to show the activation and stimulation of this system. Currently, this is one of the limitations of this research, but it is suggested that future studies investigate this issue.
The mechanisms involved in PCO caused by morphine are also not clear yet. Studies have shown that hypothalamic-pituitary-adrenal axis activity increases in PCO [33]. Some researchers believe that hypothalamic-pituitary-adrenal and sympathetic activity trigger a stress response that causes physiological and metabolic changes in the body and that the stress and elevated glucocorticoids in the blood cause oxidative stress [34].
In the present study, the concentration of glucocorticoid receptors at DH was specifically examined. However, the results showed no significant changes in glucocorticoid receptor levels at DH, identifying a questionable involvement. Therefore, further investigation is required to find out if the brain is damaged by oxidative stress processes under these conditions (PCO). The hippocampus is highly sensitive to oxidative stress [22]; however, its sensitivity to oxidative stress in the morphine-induced PCO model has not been demonstrated yet. In the present study, the level of oxidative stress in DH was higher in the morphine-treated groups with ovarian cysts, compared to the control group. Hence, morphine probably plays a role in ovarian cystogenesis by oxidative stress in DH. Nevertheless, it is not clear whether morphine is the most effective component or its metabolites.
In mammals, morphine (i.p.) is mainly metabolized in the liver by the uridine-5-phospho-glucuronosyltransferase enzyme to two glucuronide metabolites (morphine-3-glucuronide and morphine-6-glucuronide). The M3G is produced five times more than M6G, but only M6G can cross the blood-brain barrier (BBB). Moreover, M6G has been shown to cross BBB 7.5 times less than morphine. It tends to bind to the opioid μ-receptor, but due to its lower levels than morphine, its analgesic activity is significantly lower in comparison to morphine [35].
In the present study, to compare the ef6
fectiveness of morphine and its metabolites in inducing oxidative stress in the brain, an effective dose of morphine was injected into the VMH [see 16 for dose selection]. However, no significant


Figure 5. Levels of serum testosterone in all groups. Its level showed a decrease in morphine groups, compared to the control group. P<0.05 shows the difference with control based on Tukey’s post hoc.
difference was observed in the levels of oxidative stress factors in DH tissue in this group, compared to the groups that received morphine intraperitoneally.
Regarding the neuroprotective effect of baclofen, it should first be explained that almost every neuron in the hypothalamus is targeted by GABAergic synapses, including GnRH cells, β-endorphins, endogenous opioids, and even GABA itself [36]. The arcuate nucleus (ARN) is the major regulatory center for GnRH neurons. A strong anatomical circuit has been found between GABAergic neurons in ARN and GnRH neurons. Moreover, the cell body of GnRH neurons is located in the ARN, and there is no BBB in the ARN [37].
It is noteworthy that the effects of GABA on GnRH neurons secretion may differ based on the growth stage, hormonal environment, and expression of the GABA receptor subtype [38]. Baclofen, as a GABAB receptor agonist, modulates LH pulsatile secretion. Based on the results of previous studies, the GABAB receptors regulate the excitability of hypothalamic GnRH neurons [39]. These receptors are abundant in the VMH nucleus [36], which contains neurosteroid-secreting neurons. It must be mentioned that it regulates fertility and sexual acceptance [40]. This nucleus is also linked to the hippocampus [41]. Therefore, GABA receptors are found in the areas of the brain that are sensitive to sex hormones [42]. However, estrogen is not directly related to GnRH neurons. Therefore, for estrogen to affect these neurons, there must be mediating neurons. These mediating neurons are GABAergic.
In the present study, baclofen, as a GABA receptor agonist, interfered with the induction of oxidative stress. Subsequently, the oxidative stress in DH was somewhat reduced in the baclofen-treated groups, compared to the morphine-treated groups, indicating that baclofen may interfere with morphine-induced inflammation. In line with these findings, some previous studies have shown that GABA has protective effects against metabolic and reproductive disorders caused by PCOS [24]. Alavian et al. [43] have demonstrated that the injection of GABAB receptor agonist, baclofen significantly reduces morphine sensitivity expression in female rats.
Conclusions
Acute morphine is effective in inducing ovarian cystogenesis regardless of the route of administration (peripheral or central), but it induces oxidative stress in the dorsal hippocampus only in acute peripheral type. Stimulation of GABA pathways may interfere with morphine-induced cystogenesis, and baclofen may have a protective effect against the oxidative consequence of this phenomenon on the hippocampus.
Compliance with ethical guidelines
All ethical guidelines were followed and the local Ethics Committee approved this research (IR.SHAHED.REC.1399.114).
Acknowledgments
The authors would like to thank the Deputy of Research of Shahed University for supporting this research.
Authorsʼ contributions
M. K. proposed the research plan and designed the experiments. Z. J. completed the research. M. K. analyzed the data. M. Kh. and M. R. assisted in laboratory and pathology tests. All authors (M. K., Z. J., M. Kh., and M. R.) reviewed and confirmed the final manuscript prior to submission.
Funding/Support
This research has not received any grant.
Conflicts of Interest
The authors of this article have no conflicts of interest.
References
1. Ehrmann DA. Polycystic ovary syndrome. The New England Journal of Medicine. 2005; 352(12):1223-36. [DOI:10.1056/
NEJMra041536] [PMID]
2. Diamanti-Kandarakis E. PCOS in adolescents. Best Practice & Research Clinical Obstetrics & Gynaecology. 2010; 24(2):173-83. [DOI:10.1016/j.bpobgyn.2009.09.005] [PMID]
3. Hashemian Z, Afsharian P. The role of oxidative stress in polycystic ovary syndrome. Journal Of Shahid Sadoughi University Of Medical Sciences. 2020; 28(5):2635-47. [DOI:10.18502/ssu.v28i5.3972]
4. Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. The Journal of Clinical Endocrinology & Metabolism. 2005; 90(5):2810-5. [DOI:10.1210/jc.2004-2359] [PMID]
5. Goodarzi MO, Carmina E, Azziz R. Dhea, dheas and pcos. The Journal of Steroid Biochemistry and Molecular Biology. 2015; 145:213-25. [DOI:10.1016/j.jsbmb.2014.06.003] [PMID]
6. Voorhis VBJ, Dunn MS, Snyder GD, Weiner CP. Nitric oxide: an autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology. 1994; 135(5):1799-806. [DOI:10.1210/endo.135.5.7525252] [PMID]
7. Naderi T, Akbarzadeh M, Dabbaghmaneh M, Tabatabaei H. Prevalence of various phenotypes of polycystic ovarian syndrome among high school girls of Shiraz. Journal of Inflammatory Diseases. 2009; 15(4):60-7. [DOI:10.1186/
1477-7827-9-39] [PMID]
8. Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Journal of Reproductive Biology and Endocrinology. 2011; 9(1):1-7. [DOI:10.1186/1477-7827-9-39] [PMID]
9. Brennan MJ. The effect of opioid therapy on endocrine function. The American Journal of Medicine. 2013; 126(3):12-8. [DOI:10.1016/j.amjmed.2012.12.001] [PMID]
10. Katz N, Mazer NA. The impact of opioids on the endocrine system. The Clinical Journal of Pain. 2009; 25(2):170-5. [DOI:10.1097/AJP.0b013e3181850df6] [PMID]
11. Zangeneh FZ, Mohammadi A, Ejtemaeimehr S, Naghizadeh MM, Fatemeh A. The role of opioid system and its interaction with sympathetic nervous system in the processing of polycystic ovary syndrome modeling in rat. Archives of Gynecology and Obstetrics. 2011; 283(4):885-92. [DOI:10.1007/s00404-010-1776-7] [PMID]
12. Van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: an encounter of biology, psychology, and medicine. Pharmacological Reviews. 1999; 51(2):341-96. [PMID]
13. Katzung BG. Basic and clinical pharmacology.14th ed. McGraw Hill Professional; 2017.
14. Bobeck EN, Chen Q, Morgan MM, Ingram SL. Contribution of adenylyl cyclase modulation of pre- and postsynaptic GABA neurotransmission to morphine antinociception and tolerance. Neuropsychopharmacology. 2014; 39(9):2142–52. [DOI:10.1038/npp.2014.62] [PMID]
15. Karami M, Jafaryan-Dehkordi A, Darban-Fooladi M, Jalali-Nadoushan M. Induction of polycystic ovary in the rat by morphine. Daneshvar Medicine. 2015; 23(5):45-52. [DOI:10.22070/DANESHMED.2021.14147.1055]
16. Karimi R, Karami M, Jalali-Nadoushan M. Rat’s polycystic ovary due to intraventromedial hypothalamus morphine injection. Reproductive Sciences. 2018; 25(6):867-72. [DOI:10.1177/1933719117698581] [PMID]
17. Johnson K, Pinchuk I, Melgar MIE, Agwogie MO, Silva FS. The global movement towards a public health approach to substance use disorders. Annals of Medicine. 2022; 54(1):1797-808. [DOI:10.1080/07853890.2022.2079150] [PMID]
18. Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018; 43(13):2514-20. [DOI:10.1038/s41386-018-0225-3] [PMID]
19. Stefano GB, Kream RM. Dopamine, morphine, and nitric oxide: an evolutionary signaling triad. CNS Neuroscience & Therapeutics. 2010; 16(3):124-37. [DOI:10.1111/j.1755-5949.2009.00114.x] [PMID]
20. Osmanlıoğlu Ö, Yıldırım MK, Akyuva Y, Yıldızhan K, Nazıroğlu M. Morphine induces apoptosis, inflammation, and mitochondrial oxidative stress via activation of TRPM2 channel and NO signaling pathways in the hippocampus. Molecular Neurobiology. 2020; 57(8):3376-89. [DOI:10.
1007/s12035-020-01975-6] [PMID]
21. Salim S. Oxidative Stress and the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 2017; 360(1):201-5. [DOI:10.1124/jpet.116.237503] [PMID]
22. Hosseini SA, Khalilzadeh MA, Homam SM. Modeling of the behavioral calcium channels in the hippocampus cells, during stress. Iranian Journal of Biomedical Engineering. 2010; 4(1):23-31. [DOI: 10.22041/ijbme.2010.13374]
23. Liu L, Li CJ, Lu Y, Zong XG, Luo C, Sun J, et al. Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Scientific Reports. 2015; 5(1):1-17. [DOI:10.1038/srep14474] [PMID]
24. Ullah A, Jahan S, Razak S, Pirzada M, Ullah H, Almajwal A, et al. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. Journal of Ovarian Research. 2017; 10(1):1-8. [DOI:10.1186/s13048-017-0359-7] [PMID]
25. Jones KA, Tamm JA, Craig DA, Yao W-J, Panico R. Signal transduction by GABA B receptor heterodimers. Neuro-psychopharmacology. 2000; 23(1):41-9. [DOI:10.1016/S0
893-133X(00)00145-7] [PMID]
26. Khodadad A, Sani MN, Nemat-Khorasani E, Mansouri F. The effect of baclofen on treatment of infancy gastro-esophageal reflux disorder. Iranian Journal of Pediatrics. 2008; 218(1):
15-20.
27. Rashidi A, Ahmadi S. Subunits of gammaaminobutyric acid receptors and their roles in neuropsychological disorders. The Neuroscience Journal of Shefaye Khatam. 2014; 2(2):70-80. [DOI:10.18869/acadpub.shefa.2.2.70 ]
28. Listos J, Lupina M, Talarek S, Mazur A, Orzelska-Gorka J, Kotlinska J. The Mechanisms involved in morphine addiction: an overview. International Journal of Molecular Sciences. 2019;
20(17):4302. [DOI:10.3390/ijms20174302] [PMID]
29. Assadi SM, Radgoodarzi R, Ahmadi-Abhari SA. Baclofen for maintenance treatment of opioid dependence: a randomized double-blind placebo-controlled clinical trial. BMC Psychiatr. 2003; 3(1):1-10. [DOI:10.1186/1471-244X-3-16]
30. Yokoi I, Akiyama K, Kabuto H, Fukuyama K, Nishijima Y, Mori A. Peripheral acting muscle relaxants alter the effects of baclofen on the electrocorticograms in the rat. Journal of Neural Transmission. 1992; 87(1):37-47. [DOI:10.1007/
BF01253109] [PMID]
31. Salvetti NR, Canal AM, Gimeno EJ, Ortega HH. Polycystic ovarian syndrome: temporal characterization of the induction and reversion process in an experimental model. Brazilian Journal of Veterinary Research and Animal Science. 2004; 41(6):389-95. [DOI:10.1590/S1413-95962
004000600006]
32. Toda N, Kishioka S, Hatano Y, Toda H. Modulation of opioid actions by nitric oxide signaling. Anesthesiology. 2009; 110(1):166-81. [DOI: ]10.1097/ALN.0b013e3181
9146a9] [PMID]
33. Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. The Journal of Clinical Endocrinology & Metabolism. 1998; 83(7):2317-23. [DOI:10.1210/jcem.83.7.4948] [PMID]
34. Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Practice & Research Clinical Obstetrics & Gynaecology. 2016; 37:80-97. [DOI:10.1016/j.bpobgyn.2016.03.005] [PMID]
35. Gabel F, Hovhannisyan V, Berkati A-K, Goumon Y. Morphine-3-glucuronide, physiology and behavior. Frontiers in Molecular Neuroscience. 2022; 15:882443. [DOI:10.33
89/fnmol.2022.882443] [PMID]
36. Davis AM, Henion TR, Tobet SA. Gamma-aminobutyric acidB receptors and the development of the ventromedial nucleus of the hypothalamus. Journal of Comparative Neurology. 2002; 449(3):270-80. [DOI:10.1002/cne.10293] [PMID]
37. Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012; 153(11):5587-99. [DOI:10.1210/en.2012-1470] [PMID]
38. Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Frontiers in Neuroscience. 2014; 8:387. [DOI:10.3389/fnins.2014.00387] [PMID]
39. Akema T, Kimura F. Modulation of pulsatile LH secretion by baclofen, a selective GABAB receptor agonist, in ovariectomized rats. Neuroendocrinology. 1992; 56(2):141-7. [DOI:10.1159/000126221] [PMID]
40. Parandin R, Rassouli MB. Effects of endocrine disrupting compounds on hypothalamic-pituitary-gonadal axis and reproductive health A review. Iranian Journal of Endocrinology and Metabolism. 2017; 18(6):455-69.
41. Canteras N, Simerly R, Swanson L. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris‐leucoagglutinin study in the rat. Journal of Comparative Neurology. 1994; 348(1):41-79. [DOI:10.1002/cne.903480103] [PMID]
42. Bäckström T, Haage D, Löfgren M, Johansson L, Strömberg J, Nyberg S, et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 2011; 191:46-54. [DOI:10.1016/j.neuroscience.2011.03.061] [PMID]
43. Alavian F, Sahraei H, Ghiasvand S. Effects of central amygdala GABA-B on expression of morphine-induced sensitivity in female rats. Koomes. 2019; 21(2):365-373.
Regarding the neuroprotective effect of baclofen, it should first be explained that almost every neuron in the hypothalamus is targeted by GABAergic synapses, including GnRH cells, β-endorphins, endogenous opioids, and even GABA itself [36]. The arcuate nucleus (ARN) is the major regulatory center for GnRH neurons. A strong anatomical circuit has been found between GABAergic neurons in ARN and GnRH neurons. Moreover, the cell body of GnRH neurons is located in the ARN, and there is no BBB in the ARN [37].
It is noteworthy that the effects of GABA on GnRH neurons secretion may differ based on the growth stage, hormonal environment, and expression of the GABA receptor subtype [38]. Baclofen, as a GABAB receptor agonist, modulates LH pulsatile secretion. Based on the results of previous studies, the GABAB receptors regulate the excitability of hypothalamic GnRH neurons [39]. These receptors are abundant in the VMH nucleus [36], which contains neurosteroid-secreting neurons. It must be mentioned that it regulates fertility and sexual acceptance [40]. This nucleus is also linked to the hippocampus [41]. Therefore, GABA receptors are found in the areas of the brain that are sensitive to sex hormones [42]. However, estrogen is not directly related to GnRH neurons. Therefore, for estrogen to affect these neurons, there must be mediating neurons. These mediating neurons are GABAergic.
In the present study, baclofen, as a GABA receptor agonist, interfered with the induction of oxidative stress. Subsequently, the oxidative stress in DH was somewhat reduced in the baclofen-treated groups, compared to the morphine-treated groups, indicating that baclofen may interfere with morphine-induced inflammation. In line with these findings, some previous studies have shown that GABA has protective effects against metabolic and reproductive disorders caused by PCOS [24]. Alavian et al. [43] have demonstrated that the injection of GABAB receptor agonist, baclofen significantly reduces morphine sensitivity expression in female rats.
Conclusions
Acute morphine is effective in inducing ovarian cystogenesis regardless of the route of administration (peripheral or central), but it induces oxidative stress in the dorsal hippocampus only in acute peripheral type. Stimulation of GABA pathways may interfere with morphine-induced cystogenesis, and baclofen may have a protective effect against the oxidative consequence of this phenomenon on the hippocampus.
Compliance with ethical guidelines
All ethical guidelines were followed and the local Ethics Committee approved this research (IR.SHAHED.REC.1399.114).
Acknowledgments
The authors would like to thank the Deputy of Research of Shahed University for supporting this research.
Authorsʼ contributions
M. K. proposed the research plan and designed the experiments. Z. J. completed the research. M. K. analyzed the data. M. Kh. and M. R. assisted in laboratory and pathology tests. All authors (M. K., Z. J., M. Kh., and M. R.) reviewed and confirmed the final manuscript prior to submission.
Funding/Support
This research has not received any grant.
Conflicts of Interest
The authors of this article have no conflicts of interest.
References
1. Ehrmann DA. Polycystic ovary syndrome. The New England Journal of Medicine. 2005; 352(12):1223-36. [DOI:10.1056/
NEJMra041536] [PMID]
2. Diamanti-Kandarakis E. PCOS in adolescents. Best Practice & Research Clinical Obstetrics & Gynaecology. 2010; 24(2):173-83. [DOI:10.1016/j.bpobgyn.2009.09.005] [PMID]
3. Hashemian Z, Afsharian P. The role of oxidative stress in polycystic ovary syndrome. Journal Of Shahid Sadoughi University Of Medical Sciences. 2020; 28(5):2635-47. [DOI:10.18502/ssu.v28i5.3972]
4. Chhabra S, McCartney CR, Yoo RY, Eagleson CA, Chang RJ, Marshall JC. Progesterone inhibition of the hypothalamic gonadotropin-releasing hormone pulse generator: evidence for varied effects in hyperandrogenemic adolescent girls. The Journal of Clinical Endocrinology & Metabolism. 2005; 90(5):2810-5. [DOI:10.1210/jc.2004-2359] [PMID]
5. Goodarzi MO, Carmina E, Azziz R. Dhea, dheas and pcos. The Journal of Steroid Biochemistry and Molecular Biology. 2015; 145:213-25. [DOI:10.1016/j.jsbmb.2014.06.003] [PMID]
6. Voorhis VBJ, Dunn MS, Snyder GD, Weiner CP. Nitric oxide: an autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology. 1994; 135(5):1799-806. [DOI:10.1210/endo.135.5.7525252] [PMID]
7. Naderi T, Akbarzadeh M, Dabbaghmaneh M, Tabatabaei H. Prevalence of various phenotypes of polycystic ovarian syndrome among high school girls of Shiraz. Journal of Inflammatory Diseases. 2009; 15(4):60-7. [DOI:10.1186/
1477-7827-9-39] [PMID]
8. Tehrani FR, Simbar M, Tohidi M, Hosseinpanah F, Azizi F. The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Journal of Reproductive Biology and Endocrinology. 2011; 9(1):1-7. [DOI:10.1186/1477-7827-9-39] [PMID]
9. Brennan MJ. The effect of opioid therapy on endocrine function. The American Journal of Medicine. 2013; 126(3):12-8. [DOI:10.1016/j.amjmed.2012.12.001] [PMID]
10. Katz N, Mazer NA. The impact of opioids on the endocrine system. The Clinical Journal of Pain. 2009; 25(2):170-5. [DOI:10.1097/AJP.0b013e3181850df6] [PMID]
11. Zangeneh FZ, Mohammadi A, Ejtemaeimehr S, Naghizadeh MM, Fatemeh A. The role of opioid system and its interaction with sympathetic nervous system in the processing of polycystic ovary syndrome modeling in rat. Archives of Gynecology and Obstetrics. 2011; 283(4):885-92. [DOI:10.1007/s00404-010-1776-7] [PMID]
12. Van Ree JM, Gerrits MA, Vanderschuren LJ. Opioids, reward and addiction: an encounter of biology, psychology, and medicine. Pharmacological Reviews. 1999; 51(2):341-96. [PMID]
13. Katzung BG. Basic and clinical pharmacology.14th ed. McGraw Hill Professional; 2017.
14. Bobeck EN, Chen Q, Morgan MM, Ingram SL. Contribution of adenylyl cyclase modulation of pre- and postsynaptic GABA neurotransmission to morphine antinociception and tolerance. Neuropsychopharmacology. 2014; 39(9):2142–52. [DOI:10.1038/npp.2014.62] [PMID]
15. Karami M, Jafaryan-Dehkordi A, Darban-Fooladi M, Jalali-Nadoushan M. Induction of polycystic ovary in the rat by morphine. Daneshvar Medicine. 2015; 23(5):45-52. [DOI:10.22070/DANESHMED.2021.14147.1055]
16. Karimi R, Karami M, Jalali-Nadoushan M. Rat’s polycystic ovary due to intraventromedial hypothalamus morphine injection. Reproductive Sciences. 2018; 25(6):867-72. [DOI:10.1177/1933719117698581] [PMID]
17. Johnson K, Pinchuk I, Melgar MIE, Agwogie MO, Silva FS. The global movement towards a public health approach to substance use disorders. Annals of Medicine. 2022; 54(1):1797-808. [DOI:10.1080/07853890.2022.2079150] [PMID]
18. Valentino RJ, Volkow ND. Untangling the complexity of opioid receptor function. Neuropsychopharmacology. 2018; 43(13):2514-20. [DOI:10.1038/s41386-018-0225-3] [PMID]
19. Stefano GB, Kream RM. Dopamine, morphine, and nitric oxide: an evolutionary signaling triad. CNS Neuroscience & Therapeutics. 2010; 16(3):124-37. [DOI:10.1111/j.1755-5949.2009.00114.x] [PMID]
20. Osmanlıoğlu Ö, Yıldırım MK, Akyuva Y, Yıldızhan K, Nazıroğlu M. Morphine induces apoptosis, inflammation, and mitochondrial oxidative stress via activation of TRPM2 channel and NO signaling pathways in the hippocampus. Molecular Neurobiology. 2020; 57(8):3376-89. [DOI:10.
1007/s12035-020-01975-6] [PMID]
21. Salim S. Oxidative Stress and the central nervous system. Journal of Pharmacology and Experimental Therapeutics. 2017; 360(1):201-5. [DOI:10.1124/jpet.116.237503] [PMID]
22. Hosseini SA, Khalilzadeh MA, Homam SM. Modeling of the behavioral calcium channels in the hippocampus cells, during stress. Iranian Journal of Biomedical Engineering. 2010; 4(1):23-31. [DOI: 10.22041/ijbme.2010.13374]
23. Liu L, Li CJ, Lu Y, Zong XG, Luo C, Sun J, et al. Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Scientific Reports. 2015; 5(1):1-17. [DOI:10.1038/srep14474] [PMID]
24. Ullah A, Jahan S, Razak S, Pirzada M, Ullah H, Almajwal A, et al. Protective effects of GABA against metabolic and reproductive disturbances in letrozole induced polycystic ovarian syndrome in rats. Journal of Ovarian Research. 2017; 10(1):1-8. [DOI:10.1186/s13048-017-0359-7] [PMID]
25. Jones KA, Tamm JA, Craig DA, Yao W-J, Panico R. Signal transduction by GABA B receptor heterodimers. Neuro-psychopharmacology. 2000; 23(1):41-9. [DOI:10.1016/S0
893-133X(00)00145-7] [PMID]
26. Khodadad A, Sani MN, Nemat-Khorasani E, Mansouri F. The effect of baclofen on treatment of infancy gastro-esophageal reflux disorder. Iranian Journal of Pediatrics. 2008; 218(1):
15-20.
27. Rashidi A, Ahmadi S. Subunits of gammaaminobutyric acid receptors and their roles in neuropsychological disorders. The Neuroscience Journal of Shefaye Khatam. 2014; 2(2):70-80. [DOI:10.18869/acadpub.shefa.2.2.70 ]
28. Listos J, Lupina M, Talarek S, Mazur A, Orzelska-Gorka J, Kotlinska J. The Mechanisms involved in morphine addiction: an overview. International Journal of Molecular Sciences. 2019;
20(17):4302. [DOI:10.3390/ijms20174302] [PMID]
29. Assadi SM, Radgoodarzi R, Ahmadi-Abhari SA. Baclofen for maintenance treatment of opioid dependence: a randomized double-blind placebo-controlled clinical trial. BMC Psychiatr. 2003; 3(1):1-10. [DOI:10.1186/1471-244X-3-16]
30. Yokoi I, Akiyama K, Kabuto H, Fukuyama K, Nishijima Y, Mori A. Peripheral acting muscle relaxants alter the effects of baclofen on the electrocorticograms in the rat. Journal of Neural Transmission. 1992; 87(1):37-47. [DOI:10.1007/
BF01253109] [PMID]
31. Salvetti NR, Canal AM, Gimeno EJ, Ortega HH. Polycystic ovarian syndrome: temporal characterization of the induction and reversion process in an experimental model. Brazilian Journal of Veterinary Research and Animal Science. 2004; 41(6):389-95. [DOI:10.1590/S1413-95962
004000600006]
32. Toda N, Kishioka S, Hatano Y, Toda H. Modulation of opioid actions by nitric oxide signaling. Anesthesiology. 2009; 110(1):166-81. [DOI: ]10.1097/ALN.0b013e3181
9146a9] [PMID]
33. Azziz R, Black V, Hines GA, Fox LM, Boots LR. Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic-pituitary-adrenal axis. The Journal of Clinical Endocrinology & Metabolism. 1998; 83(7):2317-23. [DOI:10.1210/jcem.83.7.4948] [PMID]
34. Baskind NE, Balen AH. Hypothalamic-pituitary, ovarian and adrenal contributions to polycystic ovary syndrome. Best Practice & Research Clinical Obstetrics & Gynaecology. 2016; 37:80-97. [DOI:10.1016/j.bpobgyn.2016.03.005] [PMID]
35. Gabel F, Hovhannisyan V, Berkati A-K, Goumon Y. Morphine-3-glucuronide, physiology and behavior. Frontiers in Molecular Neuroscience. 2022; 15:882443. [DOI:10.33
89/fnmol.2022.882443] [PMID]
36. Davis AM, Henion TR, Tobet SA. Gamma-aminobutyric acidB receptors and the development of the ventromedial nucleus of the hypothalamus. Journal of Comparative Neurology. 2002; 449(3):270-80. [DOI:10.1002/cne.10293] [PMID]
37. Roa J, Herbison AE. Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology. 2012; 153(11):5587-99. [DOI:10.1210/en.2012-1470] [PMID]
38. Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Frontiers in Neuroscience. 2014; 8:387. [DOI:10.3389/fnins.2014.00387] [PMID]
39. Akema T, Kimura F. Modulation of pulsatile LH secretion by baclofen, a selective GABAB receptor agonist, in ovariectomized rats. Neuroendocrinology. 1992; 56(2):141-7. [DOI:10.1159/000126221] [PMID]
40. Parandin R, Rassouli MB. Effects of endocrine disrupting compounds on hypothalamic-pituitary-gonadal axis and reproductive health A review. Iranian Journal of Endocrinology and Metabolism. 2017; 18(6):455-69.
41. Canteras N, Simerly R, Swanson L. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris‐leucoagglutinin study in the rat. Journal of Comparative Neurology. 1994; 348(1):41-79. [DOI:10.1002/cne.903480103] [PMID]
42. Bäckström T, Haage D, Löfgren M, Johansson L, Strömberg J, Nyberg S, et al. Paradoxical effects of GABA-A modulators may explain sex steroid induced negative mood symptoms in some persons. Neuroscience. 2011; 191:46-54. [DOI:10.1016/j.neuroscience.2011.03.061] [PMID]
43. Alavian F, Sahraei H, Ghiasvand S. Effects of central amygdala GABA-B on expression of morphine-induced sensitivity in female rats. Koomes. 2019; 21(2):365-373.
Article Type: Research Article |
Subject:
Physiology
Received: 2022/09/13 | Accepted: 2023/01/2 | Published: 2023/03/19
Received: 2022/09/13 | Accepted: 2023/01/2 | Published: 2023/03/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







