Volume 8, Issue 2 (May 2021)
Avicenna J Neuro Psycho Physiology 2021, 8(2): 109-114 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Zarei M, Sarihi A, Ahmadimoghaddam D, Soltani E. Effects of Intracerebroventricular Micro-injection of Kaempferol on Anxiety: Possible GABAergic Mechanism Involved. Avicenna J Neuro Psycho Physiology 2021; 8 (2) :109-114
URL: http://ajnpp.umsha.ac.ir/article-1-301-en.html
URL: http://ajnpp.umsha.ac.ir/article-1-301-en.html
1- Department of Physiology, Neurophysiology Research Center, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran , zarei@umsha.ac.ir
2- Department of Physiology, Neurophysiology Research Center, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
3- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
2- Department of Physiology, Neurophysiology Research Center, Faculty of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
3- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Hamadan University of Medical Sciences, Hamadan, Iran
Full-Text [PDF 506 kb]
(1110 Downloads)
| Abstract (HTML) (3172 Views)
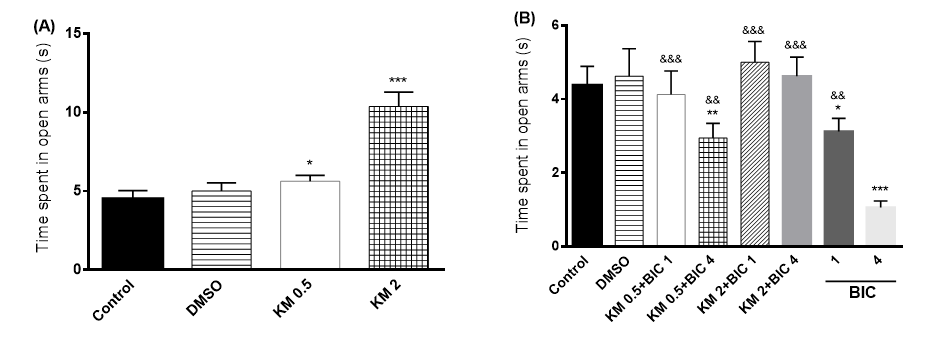
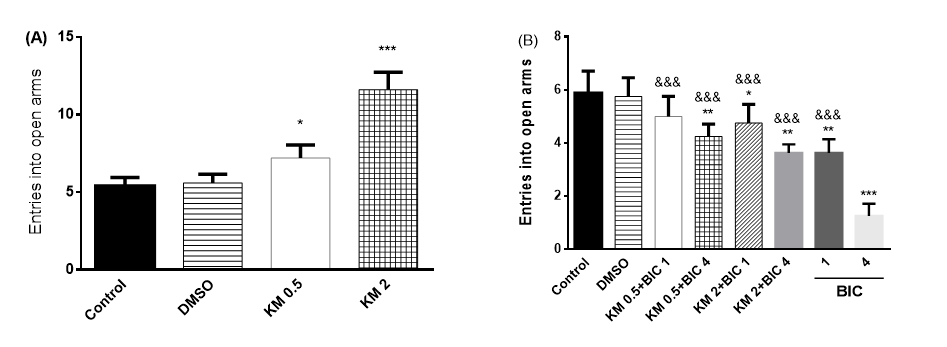
Figure 2. Effect of intracerebroventricular injection of 0.5 and 2 mg/rat of kaempferol (KM) on the entries into the open arms (A) and its interaction with GABAA mechanism (b) in male rats.
DMSO: dimethyl sulfoxide, BIC: bicuculline (1, 4 mg/rat, an antagonist of GABAA receptors). The data are expressed as mean±SEM
*: P < 0.05, **: P < 0.01, ***: P < 0.001 vs. control group
&&&: P < 0.001, vs. BIC: 4 mg/rat) (n=8 in each group)
Full-Text: (1227 Views)
Background
Today, anxiety is an overwhelming condition that is broadly associated with various disorders in the community. It is presumed that 26.9 million persons in the USA have anxiety syndromes (AS). Moreover, about 5-30% of people around the world are affected by AS during their lifetime. Predominant attainable of effective, comparatively low rate outpatient management may significantly lessen the economic and aggregate load of these common and habitually devastating disorders [1].
Presented in 1980, benzodiazepines immediately became the most generally utilized psychotropic medications. However, currently, perspectives toward these chemical compounds have extremely changed, and the development of awareness and concern about dependence risk, withdrawal marvels, and side effects have called the usage of these compounds into question [2].
The GABAA receptors (γ-Aminobutyric acid type A) are prime control elements (on the molecular levels) in light of the fact that benzodiazepines-site ligands can therapeutically attenuate anxiety. Moreover, a deficit of GABAA receptors has been recognized in the different areas of the brain in patients experiencing panic attacks. A GABAA receptors deficit has likewise been ensnared in summed up anxiety disorders [3].
Bicuculline (C2OH17NO6) is a phthalate-isoquinoline compound that can be extracted from plants, such as Dicentra cucullaria and Adlumia fungosa. Bicuculline (BIC) has also been a competitive antagonist of the GABAA ionotropic receptor. These receptors are also the most important targets of benzodiazepines and other anxiety suppressants [4, 5].
The flavonoid of Kaempferol (KM) (molecular formula: C15H10O6) is found in numerous palatable plants (e.g., tomato and grapes) or floral products frequently utilized in conventional medicine (e.g., Hedera helix and Erigeron acer) [6-8]. Some basic examinations found a positive relationship between the utilization of nourishments containing KM and a diminished risk of developing a few disorders, such as cardiovascular and neurological diseases. Various pre-clinical investigations indicated that KM as well as its glycosides have a wide range of pharmacological effects, such as anti-diabetic, anti-microbial, anti-osteoporotic, and analgesic effects [9-13]. The evidence has shown that kaempferol can act as a ligand for various receptors, such as GABAergic and cholinergic receptors [13-15].
To the best of our knowledge, no investigations have been performed on the intracerebroventricular microinjection of KM on anxiety through the GABAA mechanism.
Objectives
The present study aimed to examine the anti-anxiety effects of KM and its interaction with GABAA receptors using one of the most important validated paradigms to assess anxiety (elevated plus-maze test) in male rats.
Materials and Methods
Experimental animals
Male rodents (Wistar race rats; weighing 220–250 g) were obtained from the neurophysiology research center (animal house sub-group) of Hamadan University of Medical Sciences, Iran. All male rats were permitted to adjust to the research center conditions for about one-week for five min/day before the procedure. The rodents were kept in special boxes at standard temperature (22±2°C) with a 12/12 h dark/light cycle. The adult rats were free to use food (Pellet) or water (eight male adult rats in each laboratory group).
The present research was approved by the Ethics Committee of the Hamadan University of Medical Sciences (code: IR. UMSHA. REC. 1397. 728). In this study, the following groups (10 groups) were used (n=8 rats in each group): control, DMSO, KM 0.5 mg/rat, KM 2 mg/rat, KM 0.5 mg/rat +BIC 1 mg/rat, KM 0.5 mg/rat +BIC 4 mg/rat, KM 2 mg/rat +BIC 1 mg/rat, KM 2 mg/rat +BIC 4 mg/rat, BIC 1 mg/rat, BIC 4 mg/rat.
Medications
Kaempferol and bicuculline were bought from Sigma-Aldrich Company, USA. Dimethyl sulfoxide or DMSO were utilized for dissolving KM and BIC 20 min before intracerebroventricular micro-injection (ICVM). The KM (0.5, 2 mg/rat) and bicuculline (1, 4 mg/rat) dosages were chosen depending on recently distributed examinations [13, 16].
Surgery procedure and infusion of the medications
The surgical procedure started by co-administration of ketamine-xylazine (with the proportion of 50 mg/kg+5 mg/kg, respectively) for the production of anesthesia [17,18]. The 22-gauge tube (guide cannulas) was inserted one mm above the injection site (lateral ventricles [LV]) based on the rat brain atlas by Paxinos and Watson, bilaterally (both left and right hemisphere) [19]. The 50 μl Hamilton syringe connected to polyethylene (PE) tubing was utilized for the administration of KM or BIC. It must be mentioned that the infusion took
3-4 min.
Coordinates of the rodent brain
For this purpose, a single administration was used in the LV as follows: 0.8 mm posterior to the bregma, ±1.4 mm lateral to the sagittal suture, and 3.4 mm from the top of the skull [20, 21].
Elevated plus maze
This technique is essentially the same as the one described by Shahidi et. al [22, 23]. The elevated plus maze (EPM) consists of four arms (a wooden cross-shaped maze) and looks like a plus sign. There were two open arms as well as two closed arms in the EPM and it was elevated 50 cm. The effects of ICVM of the medications were examined in the EPM five days after cannulation. The rodents were independently positioned in the focal point of the EPM (confronting an open arm) and permitted five min of free investigation. The total time spent in the open arms (TTOA) and the number of entries into the open arms (NEOA) were determined by videotaped records.
Statistical analysis
The GraphPad Prism (Version 6) was utilized for the analysis of the significant differences among groups. Moreover, the data were scanned by ordinary one-way ANOVA and Bonferroni post-hoc test. All the results were presented as means±SEM and a p-value of less than 0.05 was considered statistically significant.
Results
According to Figure 1.A., the results of the one-way ANOVA regarding TTOA reflect the significant differences between the groups [F (3, 28) =92.4, P<0.001]. Further analysis using Bonferroni post-test revealed significant changes in the TTOA after injection of 0.5 and 2 mg/rat doses of KM, compared to the vehicle control group (P<0.05, P<0.001, respectively).
According to Figure 1.B., results of the one-way ANOVA regarding TTOA revealed a significant difference between the groups [F (7, 56) = 24.8, P < 0.001]. Further analysis using Bonferroni post-test showed that the TTOA after co-injection of KM (0.5 mg/rat) and BIC (4 mg/rat) reduced, compared to the vehicle control group (P<0.01). Moreover, there were significant differences between BIC with
Presented in 1980, benzodiazepines immediately became the most generally utilized psychotropic medications. However, currently, perspectives toward these chemical compounds have extremely changed, and the development of awareness and concern about dependence risk, withdrawal marvels, and side effects have called the usage of these compounds into question [2].
The GABAA receptors (γ-Aminobutyric acid type A) are prime control elements (on the molecular levels) in light of the fact that benzodiazepines-site ligands can therapeutically attenuate anxiety. Moreover, a deficit of GABAA receptors has been recognized in the different areas of the brain in patients experiencing panic attacks. A GABAA receptors deficit has likewise been ensnared in summed up anxiety disorders [3].
Bicuculline (C2OH17NO6) is a phthalate-isoquinoline compound that can be extracted from plants, such as Dicentra cucullaria and Adlumia fungosa. Bicuculline (BIC) has also been a competitive antagonist of the GABAA ionotropic receptor. These receptors are also the most important targets of benzodiazepines and other anxiety suppressants [4, 5].
The flavonoid of Kaempferol (KM) (molecular formula: C15H10O6) is found in numerous palatable plants (e.g., tomato and grapes) or floral products frequently utilized in conventional medicine (e.g., Hedera helix and Erigeron acer) [6-8]. Some basic examinations found a positive relationship between the utilization of nourishments containing KM and a diminished risk of developing a few disorders, such as cardiovascular and neurological diseases. Various pre-clinical investigations indicated that KM as well as its glycosides have a wide range of pharmacological effects, such as anti-diabetic, anti-microbial, anti-osteoporotic, and analgesic effects [9-13]. The evidence has shown that kaempferol can act as a ligand for various receptors, such as GABAergic and cholinergic receptors [13-15].
To the best of our knowledge, no investigations have been performed on the intracerebroventricular microinjection of KM on anxiety through the GABAA mechanism.
Objectives
The present study aimed to examine the anti-anxiety effects of KM and its interaction with GABAA receptors using one of the most important validated paradigms to assess anxiety (elevated plus-maze test) in male rats.
Materials and Methods
Experimental animals
Male rodents (Wistar race rats; weighing 220–250 g) were obtained from the neurophysiology research center (animal house sub-group) of Hamadan University of Medical Sciences, Iran. All male rats were permitted to adjust to the research center conditions for about one-week for five min/day before the procedure. The rodents were kept in special boxes at standard temperature (22±2°C) with a 12/12 h dark/light cycle. The adult rats were free to use food (Pellet) or water (eight male adult rats in each laboratory group).
The present research was approved by the Ethics Committee of the Hamadan University of Medical Sciences (code: IR. UMSHA. REC. 1397. 728). In this study, the following groups (10 groups) were used (n=8 rats in each group): control, DMSO, KM 0.5 mg/rat, KM 2 mg/rat, KM 0.5 mg/rat +BIC 1 mg/rat, KM 0.5 mg/rat +BIC 4 mg/rat, KM 2 mg/rat +BIC 1 mg/rat, KM 2 mg/rat +BIC 4 mg/rat, BIC 1 mg/rat, BIC 4 mg/rat.
Medications
Kaempferol and bicuculline were bought from Sigma-Aldrich Company, USA. Dimethyl sulfoxide or DMSO were utilized for dissolving KM and BIC 20 min before intracerebroventricular micro-injection (ICVM). The KM (0.5, 2 mg/rat) and bicuculline (1, 4 mg/rat) dosages were chosen depending on recently distributed examinations [13, 16].
Surgery procedure and infusion of the medications
The surgical procedure started by co-administration of ketamine-xylazine (with the proportion of 50 mg/kg+5 mg/kg, respectively) for the production of anesthesia [17,18]. The 22-gauge tube (guide cannulas) was inserted one mm above the injection site (lateral ventricles [LV]) based on the rat brain atlas by Paxinos and Watson, bilaterally (both left and right hemisphere) [19]. The 50 μl Hamilton syringe connected to polyethylene (PE) tubing was utilized for the administration of KM or BIC. It must be mentioned that the infusion took
3-4 min.
Coordinates of the rodent brain
For this purpose, a single administration was used in the LV as follows: 0.8 mm posterior to the bregma, ±1.4 mm lateral to the sagittal suture, and 3.4 mm from the top of the skull [20, 21].
Elevated plus maze
This technique is essentially the same as the one described by Shahidi et. al [22, 23]. The elevated plus maze (EPM) consists of four arms (a wooden cross-shaped maze) and looks like a plus sign. There were two open arms as well as two closed arms in the EPM and it was elevated 50 cm. The effects of ICVM of the medications were examined in the EPM five days after cannulation. The rodents were independently positioned in the focal point of the EPM (confronting an open arm) and permitted five min of free investigation. The total time spent in the open arms (TTOA) and the number of entries into the open arms (NEOA) were determined by videotaped records.
Statistical analysis
The GraphPad Prism (Version 6) was utilized for the analysis of the significant differences among groups. Moreover, the data were scanned by ordinary one-way ANOVA and Bonferroni post-hoc test. All the results were presented as means±SEM and a p-value of less than 0.05 was considered statistically significant.
Results
According to Figure 1.A., the results of the one-way ANOVA regarding TTOA reflect the significant differences between the groups [F (3, 28) =92.4, P<0.001]. Further analysis using Bonferroni post-test revealed significant changes in the TTOA after injection of 0.5 and 2 mg/rat doses of KM, compared to the vehicle control group (P<0.05, P<0.001, respectively).
According to Figure 1.B., results of the one-way ANOVA regarding TTOA revealed a significant difference between the groups [F (7, 56) = 24.8, P < 0.001]. Further analysis using Bonferroni post-test showed that the TTOA after co-injection of KM (0.5 mg/rat) and BIC (4 mg/rat) reduced, compared to the vehicle control group (P<0.01). Moreover, there were significant differences between BIC with

Figure 1. Effect of intracerebroventricular injection of 0.5 and 2 mg/rat of kaempferol (KM) on the TTOA (A) and its interaction with GABAA mechanism (b) in male rats.
DMSO: dimethyl sulfoxide, BIC: bicuculline (1, 4 mg/rat, an antagonist of GABAA receptors). The data are expressed as mean±SEM.
*: P < 0.05, **: P < 0.01, ***: P < 0.001 vs. control group
&&: P < 0.01, &&&: P < 0.001 vs. BIC: 4mg/rat (n=8 in each group)
DMSO: dimethyl sulfoxide, BIC: bicuculline (1, 4 mg/rat, an antagonist of GABAA receptors). The data are expressed as mean±SEM.
*: P < 0.05, **: P < 0.01, ***: P < 0.001 vs. control group
&&: P < 0.01, &&&: P < 0.001 vs. BIC: 4mg/rat (n=8 in each group)
doses of 1 and 4 mg/rat compared to the vehicle control group (P<0.05, P<0.001, respectively). In addition, the results of the co-administration
of KM (2 mg/rat)) and BIC (4 mg/rat) were significantly different from those of BIC (4 mg/rat) (P<0.001).
According to Figure 2.A., one-way ANOVA analysis of the number of entries into the open arm revealed significant differences between the groups [F (3, 16) =63.87, P<0.001]. Further analysis using Bonferroni post-test exhibited significant changes in the number of entries into the open arm after injection of 0.5 and 2 mg/rat doses of KM, compared to the vehicle control group (P<0.05, P<0.001, respectively).
According to Figure 1.B., one-way ANOVA of the number of entries into the open arm points out
the significant difference between the groups [F
(7, 56) =37.22, P< 0.001]. Further analysis using Bonferroni post-test revealed that the number of entries into the open arm after co-injection of KM (2 mg/rat) and BIC (4 mg/rat) reduced, compared to the vehicle control group (P<0.05). Moreover, there were significant differences between BIC with doses of 1 and 4 mg/rat compared to the vehicle control group (P<0.01, P<0.001, respectively). In addition, results of the co-administration of KM (2 mg/rat)) and BIC (4 mg/rat) were significantly different in comparison to those of the administration of BIC (4 mg/rat) (P<0.001).
of KM (2 mg/rat)) and BIC (4 mg/rat) were significantly different from those of BIC (4 mg/rat) (P<0.001).
According to Figure 2.A., one-way ANOVA analysis of the number of entries into the open arm revealed significant differences between the groups [F (3, 16) =63.87, P<0.001]. Further analysis using Bonferroni post-test exhibited significant changes in the number of entries into the open arm after injection of 0.5 and 2 mg/rat doses of KM, compared to the vehicle control group (P<0.05, P<0.001, respectively).
According to Figure 1.B., one-way ANOVA of the number of entries into the open arm points out
the significant difference between the groups [F
(7, 56) =37.22, P< 0.001]. Further analysis using Bonferroni post-test revealed that the number of entries into the open arm after co-injection of KM (2 mg/rat) and BIC (4 mg/rat) reduced, compared to the vehicle control group (P<0.05). Moreover, there were significant differences between BIC with doses of 1 and 4 mg/rat compared to the vehicle control group (P<0.01, P<0.001, respectively). In addition, results of the co-administration of KM (2 mg/rat)) and BIC (4 mg/rat) were significantly different in comparison to those of the administration of BIC (4 mg/rat) (P<0.001).

Figure 2. Effect of intracerebroventricular injection of 0.5 and 2 mg/rat of kaempferol (KM) on the entries into the open arms (A) and its interaction with GABAA mechanism (b) in male rats.
DMSO: dimethyl sulfoxide, BIC: bicuculline (1, 4 mg/rat, an antagonist of GABAA receptors). The data are expressed as mean±SEM
*: P < 0.05, **: P < 0.01, ***: P < 0.001 vs. control group
&&&: P < 0.001, vs. BIC: 4 mg/rat) (n=8 in each group)
Discussion
Based on the findings of this study, it can be said that intracerebroventricular microinjection of kaempferol in male rats decreases the level of experimental anxiety through the GABAA mechanism as measured by the TTOA and the NEOA of the EPM. Currently, the use of an EPM test for the evaluation of anti-anxiety medications is well established. In this test, the TTOA and the NEOA are used as indicators of anxiety [24].
According to the results of previous studies, it has been clearly shown that intrathecal injection of bicuculline (as an antagonist of GABAA-type receptors) at a dose of 4 μg reduced both the TTOA and the number of entries of female mice into the open arm [25]. Based on the findings of this study, the use of bicuculline at a dose of 4 μg could reduce anxiety which is in line with those of the previous studies. Moreover, in the present study, the use of low dose bicuculline (1 μg) was able to reduce the number of rat entries into the open arm; however, this is inconsistent with the results of previous studies in this regard. This difference could be due to the use of different routes of administration and/or dependent on the gender of rodents [26].
The results of numerous studies have confirmed the anxiolytic effect of flavonoids by intracere-broventricular injection in laboratory animals
[27-29]. For example, the low-dose of flavonoid baicalein (2 μg/rat) has been shown to exert anti-anxiety effects when injected directly into the central nervous system due to its gabaergic mechanism, which is independent of the benzodiazepine sites. In the above-mentioned study, both indicators of anxiety, NEOA and TTOA, increased in rats [30].
In this study, it was also found that the use of dehydroepiandrosterone sulfate as a GABA-A receptor antagonist could inhibit the anxiolytic effect of baicalein. The findings of this study also indicated that the use of low and high doses of KM as a flavonoid compound injected intraventricularly could increase both the NEOA and TTOA in male rats, which is inconsistent with those of previous studies. Moreover, it was found that the use of GABAA receptor antagonist (bicuculline), in a dose-dependent manner, was able to reduce the anxiolytic effect of KM, which is in line with the findings of previous research. These results indicated the anti-anxiety effect of KM through interaction with the GABAA system.
Based on the results of a study conducted by Wolfman et al. in 1994, the administration of chrysin, as a flavonoid, could reduce anxiety through GABAA receptors. According to the aforementioned study, it is clearly demonstrated that microinjection of bicuculline reversed the anti-anxiety effects of chrysin [31]. This is in line with the results of the present study which indicated that the use of bicuculline altered the anti-anxiety effects of KM. Therefore, we proposed that the anti-anxiety effects of KM may be related to interaction with GABAA receptors.
Moreover, in the previous studies, it has been demonstrated that the anxiolytic activity of KM administrated through intraperitoneal injection was partially antagonized by concomitant administration of flumazenil (a benzodiazepine antagonist), but not by WAY-100635 (5-HT1A receptor antagonist) [14]. In addition, in our laboratory, we have shown that the cholinergic system (methoctramine, a selective M2 receptor antagonist of acetylcholine) may be involved in improving the effect of KM on scopolamine-induced memory impairment [13]. Therefore, GABAaergic (neurons that produce GABA as their output) and cholinergic mechanism (s) could be involved, at least in part, in the anxiolytic activity of KM.
Studies have shown that overexpression of glyoxalase-1 (GLY) and glutathione reductase-1 (GRE) genes in the brains of rats increases anxiety-like behaviors (through oxidative stress metabolism) [32]. Due to the fact that KM contains many antioxidants, like unsaturated fatty acids, it has probably exerted its anti-anxiety effect by inhibition of the GLY and GRE genes.
As previously said, flavonoids have been reported to have strong anti-anxiety effects as well as a
wide range of pharmacological and biochemical functions. Flavonoids inhibit nitric oxide-mediated production as well as macrophage-induced nitric oxide-synthesizing enzyme mRNA expression in rats. It should also be noted that nitric oxide production also increases anxiety [33]. Therefore, in other words, the flavonoid KM may also inhibit anxiety through the nitric oxide pathway.
Flavonoids can exert their anti-anxiety effect by acting on benzodiazepine receptors attached to GABA receptors. In a study performed on Coriandrum sativum, it was found that the use of the extract of this plant due to its active constituents, KM, could reduce anxiety through the gabaergic mechanism by binding to specific sites of benzodiazepines which is consistent with the results of the present study [29].
Conclusions
In summary, the results of the present study indicated that the intracerebroventricular microinjection of KM through interaction with GABAA-A receptors could possibly improve anxiety in male rats. The cellular and molecular investigation of the lesion in specific nuclei of the brain associated with anxiety, such as the amygdala and hypothalamus as well as the study of other pathways can help us to better understand the mechanisms of anxiety altered by KM.
Compliance with ethical guidelines
The present study was approved by the Ethics Committee of the Hamadan University of Medical Sciences (IR. UMSHA. REC. 1397. 728). Moreover, the tests and methodology were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (1985, no 85-23).
Authorsʼ contributions
All authors contributed equally to the preparation of this article.
Funding/Support
This study was financed by the Vice-Chancellor for Research and Technology, Hamadan University of Medical Sciences (No: 9709286017).
Conflicts of Interest
The authors declare that there was no conflict of interest in this study.
References
Based on the findings of this study, it can be said that intracerebroventricular microinjection of kaempferol in male rats decreases the level of experimental anxiety through the GABAA mechanism as measured by the TTOA and the NEOA of the EPM. Currently, the use of an EPM test for the evaluation of anti-anxiety medications is well established. In this test, the TTOA and the NEOA are used as indicators of anxiety [24].
According to the results of previous studies, it has been clearly shown that intrathecal injection of bicuculline (as an antagonist of GABAA-type receptors) at a dose of 4 μg reduced both the TTOA and the number of entries of female mice into the open arm [25]. Based on the findings of this study, the use of bicuculline at a dose of 4 μg could reduce anxiety which is in line with those of the previous studies. Moreover, in the present study, the use of low dose bicuculline (1 μg) was able to reduce the number of rat entries into the open arm; however, this is inconsistent with the results of previous studies in this regard. This difference could be due to the use of different routes of administration and/or dependent on the gender of rodents [26].
The results of numerous studies have confirmed the anxiolytic effect of flavonoids by intracere-broventricular injection in laboratory animals
[27-29]. For example, the low-dose of flavonoid baicalein (2 μg/rat) has been shown to exert anti-anxiety effects when injected directly into the central nervous system due to its gabaergic mechanism, which is independent of the benzodiazepine sites. In the above-mentioned study, both indicators of anxiety, NEOA and TTOA, increased in rats [30].
In this study, it was also found that the use of dehydroepiandrosterone sulfate as a GABA-A receptor antagonist could inhibit the anxiolytic effect of baicalein. The findings of this study also indicated that the use of low and high doses of KM as a flavonoid compound injected intraventricularly could increase both the NEOA and TTOA in male rats, which is inconsistent with those of previous studies. Moreover, it was found that the use of GABAA receptor antagonist (bicuculline), in a dose-dependent manner, was able to reduce the anxiolytic effect of KM, which is in line with the findings of previous research. These results indicated the anti-anxiety effect of KM through interaction with the GABAA system.
Based on the results of a study conducted by Wolfman et al. in 1994, the administration of chrysin, as a flavonoid, could reduce anxiety through GABAA receptors. According to the aforementioned study, it is clearly demonstrated that microinjection of bicuculline reversed the anti-anxiety effects of chrysin [31]. This is in line with the results of the present study which indicated that the use of bicuculline altered the anti-anxiety effects of KM. Therefore, we proposed that the anti-anxiety effects of KM may be related to interaction with GABAA receptors.
Moreover, in the previous studies, it has been demonstrated that the anxiolytic activity of KM administrated through intraperitoneal injection was partially antagonized by concomitant administration of flumazenil (a benzodiazepine antagonist), but not by WAY-100635 (5-HT1A receptor antagonist) [14]. In addition, in our laboratory, we have shown that the cholinergic system (methoctramine, a selective M2 receptor antagonist of acetylcholine) may be involved in improving the effect of KM on scopolamine-induced memory impairment [13]. Therefore, GABAaergic (neurons that produce GABA as their output) and cholinergic mechanism (s) could be involved, at least in part, in the anxiolytic activity of KM.
Studies have shown that overexpression of glyoxalase-1 (GLY) and glutathione reductase-1 (GRE) genes in the brains of rats increases anxiety-like behaviors (through oxidative stress metabolism) [32]. Due to the fact that KM contains many antioxidants, like unsaturated fatty acids, it has probably exerted its anti-anxiety effect by inhibition of the GLY and GRE genes.
As previously said, flavonoids have been reported to have strong anti-anxiety effects as well as a
wide range of pharmacological and biochemical functions. Flavonoids inhibit nitric oxide-mediated production as well as macrophage-induced nitric oxide-synthesizing enzyme mRNA expression in rats. It should also be noted that nitric oxide production also increases anxiety [33]. Therefore, in other words, the flavonoid KM may also inhibit anxiety through the nitric oxide pathway.
Flavonoids can exert their anti-anxiety effect by acting on benzodiazepine receptors attached to GABA receptors. In a study performed on Coriandrum sativum, it was found that the use of the extract of this plant due to its active constituents, KM, could reduce anxiety through the gabaergic mechanism by binding to specific sites of benzodiazepines which is consistent with the results of the present study [29].
Conclusions
In summary, the results of the present study indicated that the intracerebroventricular microinjection of KM through interaction with GABAA-A receptors could possibly improve anxiety in male rats. The cellular and molecular investigation of the lesion in specific nuclei of the brain associated with anxiety, such as the amygdala and hypothalamus as well as the study of other pathways can help us to better understand the mechanisms of anxiety altered by KM.
Compliance with ethical guidelines
The present study was approved by the Ethics Committee of the Hamadan University of Medical Sciences (IR. UMSHA. REC. 1397. 728). Moreover, the tests and methodology were in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (1985, no 85-23).
Authorsʼ contributions
All authors contributed equally to the preparation of this article.
Funding/Support
This study was financed by the Vice-Chancellor for Research and Technology, Hamadan University of Medical Sciences (No: 9709286017).
Conflicts of Interest
The authors declare that there was no conflict of interest in this study.
References
- Hare BD, Duman RS. Prefrontal cortex circuits in depression and anxiety: contribution of discrete neuronal populations and target regions. Molecular Psychiatry. 2020; 25(11):2742-58. [DOI:10.1038/s41380-020-0685-9] [PMID] [PMCID]
- Akter S, Uddin KR, Sasaki H, Lyu Y, Shibata S. Gamma oryzanol impairs alcohol-induced anxiety-like behavior in mice via upregulation of central monoamines associated with Bdnf and Il-1β signaling. Scientific Reports. 2020; 10(1):10677. [DOI:10.1038/s41598-020-67689-w] [PMID] [PMCID]
- Xiao Q, Zhou X, Wei P, Xie L, Han Y, Wang J, et al. A new GABAergic somatostatin projection from the BNST onto accumbal parvalbumin neurons controls anxiety. Molecular Psychiatry. 2020; In Press. [DOI:10.1038/s41380-020-0816-3] [PMID]
- Cueto-Escobedo J, Andrade-Soto J, Lima-Maximino M, Maximino C, Hernández-López F, Rodríguez-Land JF. Involvement of GABAergic system in the antidepressant-like effects of chrysin (5, 7-dihydroxyflavone) in ovariectomized rats in the forced swim test: comparison with neurosteroids. Behavioural Brain Research. 2020; 386:112590. [DOI: 10.1016/j.bbr.2020.112590] [PMID]
- Mohammadi S, Oryan S, Komaki A, Eidi A, Zarei M. Effects of hippocampal microinjection of irisin, an exercise-induced myokine, on spatial and passive avoidance learning and memory in male rats. International Journal of Peptide Research and Therapeutics. 2020; 26(1): 357-67.
- Mahmoudi M, Shahidi S, Golmohammadi H, Mohammadi S. The effect of Echium amoenum hydro-alcoholic extract on blood glucose level, lipid profile and lipoproteins in Streptozotocin-induced diabetic male rats. Journal of Zanjan University of Medical Sciences and Health Services. 2015; 23(97):72-81.
- Asgari Nematian M, Yaghmaei P, Mohammadi S. Assessment of the antinociceptive, antiinflammatory and acute toxicity effects of Ducrosia anethifolia essential oil in mice. Sci J Kurdistan Univ Med Sci. 2017; 22:74-84.
- Fallahzadeh AR, Zarei M, Mohammadi S. Preliminary phytochemical screening, analgesic and anti-inflammatory effect of Eryngium pyramidale Boiss. & Husson essential oil in male rat. Entomology and Applied Science Letters. 2016; 3(5):140-7.
- Calderon-Montano JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Reviews in Medicinal Chemistry. 2011; 11(4):298-344. [DOI:10.2174/138955711795305335] [PMID]
- Chen AY, Chen YC. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chemistry. 2013; 138(4):2099-107. [DOI:10.1016/
j.foodchem.2012.11.139] [PMID] [PMCID] - Moore W, Alkhalidy H, Zhou K, Liu D. Flavonol kaempferol improves glucose homeostasis via suppressing hepatic glucose production and enhancing insulin sensitivity in diabetic mice. The FASEB Journal. 2017; 31(1 Suppl):646-52. [DOI:10.1096/fasebj.31.1_supplement.646.52]
- Golshani Y, Mohammadi S. Evaluation of antinociceptive effect of methanolic extract of Lallemantia iberica in adult male rats. Armaghane danesh. 2015; 19(12):1058-68.
- Zarei M, Mohammadi S, Jabbari S, Shahidi S. Intracerebroventricular microinjection of kaempferol on memory retention of passive avoidance learning in rats: involvement of cholinergic mechanism (s). International Journal of Neuroscience. 2019; 129(12):1203-12. [DOI: 10.1080/00207454.2019.1653867] [PMID]
- Grundmann O, Nakajima JI, Kamata K, Seo S, Butterweck V. Kaempferol from the leaves of Apocynum venetum possesses anxiolytic activities in the elevated plus maze
test in mice. Phytomedicine. 2009; 16(4):295-302. [DOI:10.1016/j.phymed.2008.12.020] [PMID] - Aguirre-Hernández E, González-Trujano ME, Terrazas T, Santoyo JH, Guevara-Fefer P. Anxiolytic and sedative-like effects of flavonoids from Tilia americana var. mexicana: GABAergic and serotonergic participation. Salud Mental. 2016; 39(1):37-46. [DOI:10.17711/SM.0185-3325.2015.066]
- Gastón MS, Cid MP, Salvatierra NA. Bicuculline, a GABAA-receptor antagonist, blocked HPA axis activation induced by ghrelin under an acute stress. Behavioural Brain Research. 2017; 320:464-72. [DOI:10.1016/j.bbr.2016.10.035] [PMID]
- Eidi M, Zarrindast MR, Eidi A, Oryan S, Parivar K. Effects of histamine and cholinergic systems on memory retention of passive avoidance learning in rats. European Journal of Pharmacology. 2003; 465(1-2):91-6. [DOI:10.1016/s0014-2999(03)01440-7] [PMID]
- Golshani Y, Mohammadi S. Effects of Rhus Coriaria essential oil on depression and anxiety in male rats. Feyz Journal. 2019; 23(5):476-84.
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates in stereotaxic coordinates. Amsterdam, Netherlands: Elsevier; 2007.
- Shekarian M, Komaki A, Shahidi S, Sarihi A, Salehi I, Raoufi S. The protective and therapeutic effects of vinpocetine, a PDE1 inhibitor, on oxidative stress and learning and memory impairment induced by an intracerebroventricular (ICV) injection of amyloid beta (aβ) peptide. Behavioural Brain Research. 2020; 383:112512. [DOI:10.1016/j.bbr.
2020.112512] [PMID] - Mohammadi S, Oryan S, Komaki A, Eidi A, Zarei M. Effects of intra-dentate gyrus microinjection of myokine irisin on long-term potentiation in male rats. Arquivos de Neuropsiquiatria. 2019; 77(12):881-7. [DOI:10.1590/0004-282X20190184] [PMID]
- Shahidi S, Hashemi-Firouzi N, Mahmoodi M. Co-administration of fluoxetine and Sildenafil has benefits in anxiety behavior in mice. Neurochemical Journal. 2013; 7(1):34-8. [DOI:10.1134/S181971241301008X]
- Zarei M, Ahmadimoghaddam D, Mohammadi S. Artemisia biennis Willd.: Anti-Nociceptive effects and possible mechanisms of action. Journal of Ethnopharmacology. 2021; 268: 113604. [DOI: 10.1016/j.jep.2020.113604]
- Mahmoodi M, Mohammadi S, Enayati F. Evaluation of the antinociceptive effect of hydroalcoholic extract of Potentilla reptans L. in the adult male rat. SSU_Journals. 2016; 24(3):201-10.
- Zarrindast MR, Rostami P, Sadeghi-Hariri M. GABAA but not GABAB receptor stimulation induces antianxiety profile in rats. Pharmacology Biochemistry and Behavior. 2001; 69(1-2):9-15. [DOI:10.1016/s0091-3057(01)00518-4] [PMID]
- Lawton CA, Kallai J. Gender differences in wayfinding strategies and anxiety about wayfinding: A cross-cultural comparison. Sex Roles. 2002; 47(9-10):389-401. [DOI:10.1023/A:1021668724970]
- Zhang LM, Yao JZ, Li Y, Li K, Chen HX, Zhang YZ, et al. Anxiolytic effects of flavonoids in animal models of posttraumatic stress disorder. Evidence-Based Complementary and Alternative Medicine. 2012; 2012:623753. [DOI: 10.1155/2012/623753] [PMID] [PMCID]
- Lv YW, Guo JY, Liu Y, Liu J, Wu MX, He YS, et al. Advanced in studies on anxiolytic effects of natural flavonoids. Zhongguo Zhong Yao Za Zhi. 2016; 41(1):38-44. [DOI:10.4268/cjcmm20160108] [PMID]
- Emamghoreishi M, Khasaki M, Aazam MF. Coriandrum sativum: evaluation of its anxiolytic effect in the elevated plus-maze. Journal of Ethnopharmacology. 2005; 96(3):365-70. [DOI:10.1016/j.jep.2004.06.022] [PMID]
- de Carvalho RSM, Duarte FS, de Lima TCM. Involvement of GABAergic non-benzodiazepine sites in the anxiolytic-like and sedative effects of the flavonoid baicalein in mice. Behavioural Brain Research. 2011; 221(1):75-82. [DOI:10.1016/j.bbr.2011.02.038] [PMID]
- Wolfman C, Viola H, Paladini A, Dajas F, Medina JH. Possible anxiolytic effects of chrysin, a central benzodiazepine receptor ligand isolated from Passiflora coerulea. Pharmacology Biochemistry and Behavior. 1994; 47(1):1-4. [DOI:10.1016/0091-3057(94)90103-1] [PMID]
- Rosa JM, Dafre AL, Rodrigues ALS. Antidepressant-like responses in the forced swimming test elicited by glutathione and redox modulation. Behavioural Brain Research. 2013; 253:165-72. [DOI:10.1016/j.bbr.2013.07.009] [PMID]
- García-Mediavilla V, Crespo I, Collado PS, Esteller A, Sánchez-Campos S, Tuñón MJ, et al. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. European Journal of Pharmacology. 2007; 557(2-3):221-9. [DOI: 10.1016/j.ejphar.2006.11.014] [PMID]
Article Type: Research Article |
Subject:
Anxiety and Stress
Received: 2020/08/7 | Accepted: 2020/09/14 | Published: 2021/05/20
Received: 2020/08/7 | Accepted: 2020/09/14 | Published: 2021/05/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







