Volume 12, Issue 2 (June 2025)
Avicenna J Neuro Psycho Physiology 2025, 12(2): 89-95 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Taiebine M. Neuropsychological Profile of Topographical Disorientation: Case Report with Review of Literature. Avicenna J Neuro Psycho Physiology 2025; 12 (2) :89-95
URL: http://ajnpp.umsha.ac.ir/article-1-522-en.html
URL: http://ajnpp.umsha.ac.ir/article-1-522-en.html
Euromed Research Center, Université Euro Méditerranéenne de Fès (UEMF), Fès, Morocco. , m.taiebine@ueuromed.org
Full-Text [PDF 281 kb]
(252 Downloads)
| Abstract (HTML) (871 Views)
Table 1. Neuropsychological assessment of MB in 2021
Full-Text: (190 Views)
Background
The neuropsychological investigation of spatial cognition focuses on the brain's functional architecture and neurocognitive components that facilitate spatial perception [1]. In this context, cognitive maps examine the significance of place in the genesis and storage of spatial representations within the brain, which is crucial for understanding memory processes and the dynamics of healthy aging [2].
Regarding spatial cognition, it encompasses a network of brain regions across different species, characterized by a fundamental network for spatial processing that includes symmetrically arranged areas in both hemispheres. This network features the dorsal fronto-parietal regions, the pre-supplementary motor area, the anterior insula, and the frontal operculum [3]. Moreover, specific neuronal networks within the occipital and parietal lobes are specialized in the localization of objects, detection of line orientation, and spatial synthesis. Furthermore, the striatum, which has a close relationship with the hippocampus, plays a significant role in spatial cognition by integrating both external and internal information [3].
On the one hand, topographical orientation is significantly shaped by factors, such as visual attention, spatial working memory, and visuospatial perception, which engage multiple brain regions. Different authors indicate that the posterior hippocampus of London taxi drivers is larger compared to that of average drivers, a difference attributed to their necessity to memorize street names and routes [4, 5]. Neuroimaging investigations have demonstrated that the parieto-occipital regions are responsible for processing visual flow, the parahippocampal gyrus plays a role in recognizing spatial cues, and the retrosplenial cortex is involved in directional orientation [6].
On the other hand, disorders related to topographical memory, often referred to as spatial agnosia, manifest as challenges in recognizing and navigating through spatial environments [7]. Individuals affected by these disorders may find it challenging to recall routes, identify landmarks, or orient themselves within familiar surroundings. Furthermore, those with amnestic mild cognitive impairment (a-MCI) and mild Alzheimer's disease (AD) show deficits in topographical short-term memory, underscoring the vulnerability of this cognitive function during the early phases of AD [8]. These disorders are generally associated with impairments in various cognitive abilities, including attention, working memory, planning, and spatial perception. Therefore, evaluating topographical memory disorders is crucial for the diagnosis and management of conditions linked to cognitive decline and neurodegenerative diseases.
In terms of cognitive modelling of spatial cognition, Aguirre and D'Esposito [9] suggested a taxonomy and theoretical framework including egocentric disorientation. They distinguished between allocentric and egocentric spatial viewpoints, which are fundamental to spatial navigation and orientation. The egocentric system represents a "self-centered" frame of reference [9]. It encodes the positions of objects and landmarks in relation to the body. For instance, an egocentric map indicates that the coffee shop is "to the right of the observer.” This form of processing is crucial for immediate actions and moment-to-moment navigation, such as reaching for a cup or dodging obstacles. It is the adopted viewpoint while someone is actively traversing a space.
In contrast, the allocentric system serves as an "environment-centered" or "world-centered" frame of reference [9]. It encodes the locations of objects relative to one another, independent of the observer’s position. This system enables us to comprehend that the coffee shop is "situated between the park and the bookstore," irrespective of the standing position. Allocentric processing is essential for long-term spatial memory, planning intricate routes, and retracing steps to a location after an extended period of time. This issue is what causes an individual with topographical disorientation (TD) to struggle with a "blind map task," as they are unable to access this internal, viewpoint-independent representation of the environment.
Objectives
This single case study focused on characterizing the neuropsychological profile of topographical memory disorder in Mrs. MB, a Moroccan French-speaking patient who expressed considerable challenges in navigating familiar locations in Rabat (capital of Morocco).
Case Presentation
Mrs. MB, an 81-year-old right-handed woman with a French Baccalaureate, sought a neuropsychological evaluation due to complaints of TD and navigational memory difficulties. Despite her concerns, she remains independent, drives, and engages in regular sports and yoga, thus demonstrating a high level of daily functioning.
The evaluation revealed mild, non-progressive memory fixation disorders that did not affect her daily activities. Although memory complaints are frequently observed in individuals with TD, the non-progressive aspect of her fixation symptoms is unusual compared to the typical memory decline associated with early AD. In general, memory deficits in AD are progressive and tend to worsen over time. This issue indicates that her memory challenges may not be directly related to the same underlying pathology as her pronounced TD, or that the progression of her AD is notably slow.
She also exhibited a slight tremor in her hands and experienced bilateral hearing impairment. Neuroimaging of the brain using MRI indicated enlargement of the parietal sulci, dilation of the posterior ventricles, and the presence of vascular lacunae within the white matter. Importantly, her hippocampal volume was normal.
Results
The neuropsychological assessment (Table 1) of Mrs. MB identified normal functioning of global cognitive functions, as indicated by the Montreal Cognitive Assessment (MoCA) and Progressive Matrices 47 (PM 47) tests. Executive and attentional functions did not show any abnormalities. However, it is important to highlight that the processes involved in encoding and retrieving information in semantic and episodic memory did not show critical issues. There was an observable learning effect, accompanied by slight executive impairment, which was normalized by cued recall. Such performance did not show a hippocampal profile. While gestural, visuo-perceptual (absence of asemantic and apperceptive agnosia), visuo-spatial, and visuo-constructional abilities remain intact, there was a marked impairment in visual navigational tasks.
Regarding spatial cognition, it encompasses a network of brain regions across different species, characterized by a fundamental network for spatial processing that includes symmetrically arranged areas in both hemispheres. This network features the dorsal fronto-parietal regions, the pre-supplementary motor area, the anterior insula, and the frontal operculum [3]. Moreover, specific neuronal networks within the occipital and parietal lobes are specialized in the localization of objects, detection of line orientation, and spatial synthesis. Furthermore, the striatum, which has a close relationship with the hippocampus, plays a significant role in spatial cognition by integrating both external and internal information [3].
On the one hand, topographical orientation is significantly shaped by factors, such as visual attention, spatial working memory, and visuospatial perception, which engage multiple brain regions. Different authors indicate that the posterior hippocampus of London taxi drivers is larger compared to that of average drivers, a difference attributed to their necessity to memorize street names and routes [4, 5]. Neuroimaging investigations have demonstrated that the parieto-occipital regions are responsible for processing visual flow, the parahippocampal gyrus plays a role in recognizing spatial cues, and the retrosplenial cortex is involved in directional orientation [6].
On the other hand, disorders related to topographical memory, often referred to as spatial agnosia, manifest as challenges in recognizing and navigating through spatial environments [7]. Individuals affected by these disorders may find it challenging to recall routes, identify landmarks, or orient themselves within familiar surroundings. Furthermore, those with amnestic mild cognitive impairment (a-MCI) and mild Alzheimer's disease (AD) show deficits in topographical short-term memory, underscoring the vulnerability of this cognitive function during the early phases of AD [8]. These disorders are generally associated with impairments in various cognitive abilities, including attention, working memory, planning, and spatial perception. Therefore, evaluating topographical memory disorders is crucial for the diagnosis and management of conditions linked to cognitive decline and neurodegenerative diseases.
In terms of cognitive modelling of spatial cognition, Aguirre and D'Esposito [9] suggested a taxonomy and theoretical framework including egocentric disorientation. They distinguished between allocentric and egocentric spatial viewpoints, which are fundamental to spatial navigation and orientation. The egocentric system represents a "self-centered" frame of reference [9]. It encodes the positions of objects and landmarks in relation to the body. For instance, an egocentric map indicates that the coffee shop is "to the right of the observer.” This form of processing is crucial for immediate actions and moment-to-moment navigation, such as reaching for a cup or dodging obstacles. It is the adopted viewpoint while someone is actively traversing a space.
In contrast, the allocentric system serves as an "environment-centered" or "world-centered" frame of reference [9]. It encodes the locations of objects relative to one another, independent of the observer’s position. This system enables us to comprehend that the coffee shop is "situated between the park and the bookstore," irrespective of the standing position. Allocentric processing is essential for long-term spatial memory, planning intricate routes, and retracing steps to a location after an extended period of time. This issue is what causes an individual with topographical disorientation (TD) to struggle with a "blind map task," as they are unable to access this internal, viewpoint-independent representation of the environment.
Objectives
This single case study focused on characterizing the neuropsychological profile of topographical memory disorder in Mrs. MB, a Moroccan French-speaking patient who expressed considerable challenges in navigating familiar locations in Rabat (capital of Morocco).
Case Presentation
Mrs. MB, an 81-year-old right-handed woman with a French Baccalaureate, sought a neuropsychological evaluation due to complaints of TD and navigational memory difficulties. Despite her concerns, she remains independent, drives, and engages in regular sports and yoga, thus demonstrating a high level of daily functioning.
The evaluation revealed mild, non-progressive memory fixation disorders that did not affect her daily activities. Although memory complaints are frequently observed in individuals with TD, the non-progressive aspect of her fixation symptoms is unusual compared to the typical memory decline associated with early AD. In general, memory deficits in AD are progressive and tend to worsen over time. This issue indicates that her memory challenges may not be directly related to the same underlying pathology as her pronounced TD, or that the progression of her AD is notably slow.
She also exhibited a slight tremor in her hands and experienced bilateral hearing impairment. Neuroimaging of the brain using MRI indicated enlargement of the parietal sulci, dilation of the posterior ventricles, and the presence of vascular lacunae within the white matter. Importantly, her hippocampal volume was normal.
Results
The neuropsychological assessment (Table 1) of Mrs. MB identified normal functioning of global cognitive functions, as indicated by the Montreal Cognitive Assessment (MoCA) and Progressive Matrices 47 (PM 47) tests. Executive and attentional functions did not show any abnormalities. However, it is important to highlight that the processes involved in encoding and retrieving information in semantic and episodic memory did not show critical issues. There was an observable learning effect, accompanied by slight executive impairment, which was normalized by cued recall. Such performance did not show a hippocampal profile. While gestural, visuo-perceptual (absence of asemantic and apperceptive agnosia), visuo-spatial, and visuo-constructional abilities remain intact, there was a marked impairment in visual navigational tasks.
Table 1. Neuropsychological assessment of MB in 2021
| Tests | Initial Assessment in 2021 | Cut-off Score (Approximate for 80-84 years, Based on Typical Norms) | Interpretation of Mrs. MB's Score |
| MoCA (Montreal Cognitive Assessment) | Total Score: 26/30 | ≤23/30 (MCI) | Mrs. MB's score of 26/30 is within the normal range for her age, suggesting overall preserved global cognitive function. |
| Visuospatial/Executive 5/5 Naming 3/3 Attention 5/5 Language (Fluency: 19) 3/3 (Fluency: 19) Abstraction 2/2 Delayed Recall 2/5 Orientation 6/6 |
|||
| PM 47 (Progressive Matrices 47) | Set 1: 11/12; Set 2: 8/12; Set 3: 10/12; Total score: 29/36; Time: 14 min 45 sec |
≤24/36 (Mild Impairment) | Her total score is above the typical cut-off, indicating intact non-verbal reasoning abilities for her age. |
| FAB (Frontal Assessment Battery) | Total Score: 18/18 | ≤13/18 (Mild Frontal Dysfunction) | A perfect score suggests excellent frontal executive function, including conceptualization, mental flexibility, and inhibitory control. |
| Conceptualization 3/3 Mental Flexibility 3/3 Motor Programming 3/3 Sensitivity to Interference 3/3 Inhibitory Control 3/3 Environmental Autonomy 3/3 |
|||
| TMT - Trail Making Test | TMT A (0 Errors/33 sec); TMT B (0 Errors/1 min 11 s) |
TMT A: ≤40−50 sec; TMT B: ≤120−150 sec | Excellent performance on both parts, indicating preserved visual attention, sequencing, and cognitive flexibility. |
| Cancellation Test – Bells Test | Total Number of Bells Circled: 32/35 | ≥28−30/35 (Normal) | Performance is within the normal range for visual scanning and selective attention. |
| Clock Drawing Test | 10/10 | ≥7/10 (Normal) | A perfect score indicates intact visuospatial organization and executive planning. |
| Categorical and Literal Verbal Fluency | Letter Fluency (P=16) (0 Intrusions)/(R=18) (3 Intrusions); Semantic Category Fluency: Animals (21) 0 Repetitions/2 Intrusions; Fruits (15) 1 Intrusion/0 Repetitions |
Letter Fluency (P, R): ≥9−12; Semantic Fluency (Animals, Fruits): ≥14−18 | Strong performance on both letter and semantic fluency, indicating good verbal retrieval. |
| Hayling Test | Part 1: 15/15; Part 2: 15/15 |
Part 1: ≥13/15; Part 2: ≥13/15 | Perfect scores suggest preserved initiation and suppression abilities. |
| Digit Span Task | Digit Span Forwards (6); Digit Span Backwards (5); Visual Span: 5 | Forwards: ≥5−6; Backwards: ≥4−5; Visual: ≥4−5 | All scores are within the normal range for auditory and visual working memory span. |
| FCSRT (Free and Cued Selective Reminding Test) | Immediate Recall: 13/16; Recall 1: Free Recall 4/16, Cued Recall 7/16, Total Recall: 11/16 (1 Intrusion); Recall 2: Free Recall 7/16, Cued Recall 8/16, Total Recall: 15/16 (1 Intrusion); Recall 3: Free Recall 9/16, Cued Recall 7/16, Total Recall: 16/16; Free Delayed Recall: 8/16, Cued Delayed Recall: 8/16, Total: 16/16; Recognition: 16/16 |
Immediate Recall: ≥12/16; Free Delayed Recall: ≥10/16; Total Recall (With Cueing): ≥14/16; Recognition: ≥15/16 | Immediate recall is good. However, her free delayed recall (8/16) is significantly impaired, which is characteristic of hippocampal-based memory deficits. Cued recall largely improves her performance, suggesting a retrieval rather than encoding deficit, but it does not fully normalize, indicating a very mild storage issue. This pattern is often seen in early AD. Recognition is preserved. |
| PEGV (Protocol Montreal Toulouse for the exploration of Gnosia) | Intertwined Figures: 34/36; Identical Figures: 12/12; Categorical Matching: 7/10; Functional Matching: 10/10 |
Intertwined Figures: ≥30/36; Identical Figures: ≥9/12; Categorical Matching: ≥8/10; Functional Matching: ≥9/10 | Intertwined and identical figures are good. However, her categorical matching (7/10) is mildly impaired, suggesting some difficulty with semantic categorization. |
| VOSP (Visual Object and Space Perception) | Incomplete Letters: 19/20; Silhouettes: Animals 6/15; objects 5/15; Total: 11/30; Object Decision: 16/20; Progressive Silhouettes: 12/20; Dot Counting: 10/10; Position Discrimination: 19/20; Number Location: 10/10; Cube Analysis: 7/10 |
Incomplete Letters: ≥18/20; Silhouettes: ≥20/30; Object Decision: ≥18/20; Progressive Silhouettes: ≥16/20; Dot Counting: ≥9/10; Position Discrimination: ≥18/20; Number Location: ≥9/10; Cube Analysis: ≥8/10 | Significant impairments are marked in 'Silhouettes' (11/30), 'Object decision' (16/20), and 'Progressive silhouettes' (12/20), which are direct measures of visual object recognition and perceptual abilities. This is highly relevant to her TD, as these tasks assess foundational visual processing, which is often disrupted in posterior cortical atrophy (PCA). Other VOSP sub-tests are generally preserved. |
| Praxis Examination | Symbolic Gestures 5/5; Pantomimes 10/10; Abstract Gestures 8/8 |
All Sub-tests: ≥4/5, ≥9/10, ≥7/8 | Fully preserved praxis, indicating no difficulties with motor planning or execution of learned movements. |
| ROCF (The Rey-Osterrieth Complex Figure) Test | Total Score: 34/36; Time: 6 min 5 s | ≥28/36 (Normal) | Her excellent score indicates preserved visuospatial constructional abilities and organizational strategy. |
| IADL (Instrumental Activities of Daily Living) | 8/8 | ≥8/8 (Independent) | Fully independent in instrumental activities of daily living. |
Mrs. MB's neuropsychological assessment revealed several cognitive strengths and weaknesses. Although her MoCA and FAB scores, in conjunction with the TMT and Clock Drawing, indicated maintained global cognitive function and executive capabilities. This inconsistency highlights the varying sensitivities of different cognitive screening instruments. Importantly, the notable impairments observed in the VOSP 'Silhouettes' and 'Progressive Silhouettes' subtests were directly linked to her reported TD.
|
These assessments evaluate visual object recognition and the capacity to synthesize fragmented visual information, which are essential for identifying landmarks and constructing a coherent mental representation of space (allocentric spatial processing). Such deficits are highly indicative of PCA, a neurodegenerative condition frequently associated with Alzheimer's pathology, in which visuospatial and perceptual deficits are the primary presenting symptoms, even when memory for daily events or executive functions seems relatively intact. The slight impairment in free delayed recall on the FCSRT aligns with the underlying memory deficit commonly seen in AD. This issue raises the possibility that her memory issues may be less severe than anticipated, given the extent of her visuospatial impairment, or that she may represent a distinctive atypical clinical presentation.It is important to pinpoint that PCA presents a unique neurocognitive profile. Positive signs indicate impairments in higher-level visual cognition, leading to difficulties in interpreting what is observed (moderately impaired in our patient). However, negative signs refer to the preservation of basic visual, gestural, and visuo-constructional abilities. This distinction between what is lost and what remains intact is key to diagnosing PCA and differentiating it from other neurodegenerative disorders.
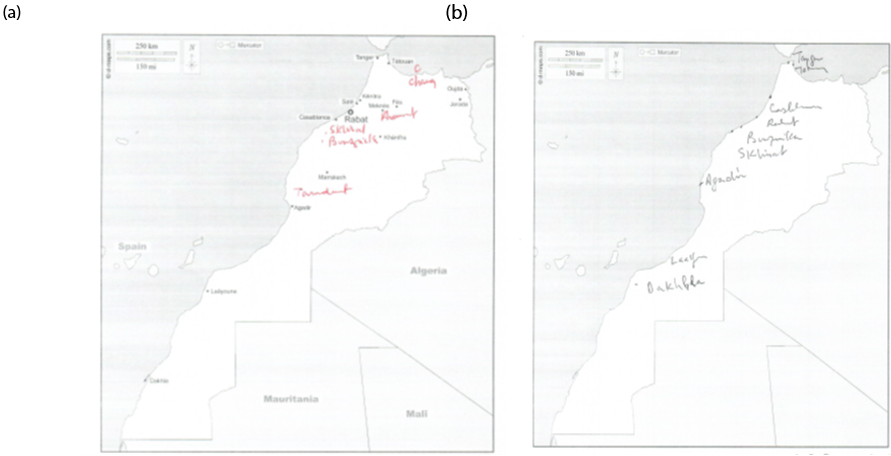
Regarding navigational tasks, they were assessed through cognitive tasks that involved positioning landmarks on a map of a specific city in Morocco, as well as indicating directions to different locations from the individual's current location, which were impaired (Figure 1).
Regarding navigational tasks, they were assessed through cognitive tasks that involved positioning landmarks on a map of a specific city in Morocco, as well as indicating directions to different locations from the individual's current location, which were impaired (Figure 1).

Figure 1. Comparing patient’s performance in spatial cognition tasks–– (a) map with points of secondary cities not designated; (b) blind map of Morocco (located cities).
Discussion
Given the aforementioned results, it is clear that Mrs MB displays a specific TD. In addition to the medial temporal lobe, TD may indicate atrophy in the parietal regions, which are essential for spatial processing. Such atrophy in these parietal areas can hinder the integration of sensory information and the creation of mental spatial representations, leading to challenges in navigation, performing blind map tasks, and allocentric spatial memory [10]. These tasks require individuals to place landmarks on a map without visual aids, depending solely on their mental constructs of spatial relationships [11].
The neurocognitive profile displayed by Mrs. MB highlights that her particular TD extends beyond mere age-related forgetfulness, as it encompasses a profound inability to orient herself, even within familiar settings. This observation aligns with the trend of cognitive decline, where spatial abilities are disproportionately affected compared to other cognitive functions, which is essential for differentiating it from general cognitive deterioration or other types of dementia. Although TD may arise from various neurological injuries, such as stroke or traumatic brain injury, its occurrence without notable cognitive deficits can act as an early indicator of AD or an atypical visual variant of AD, including PCA [12].
The MRI findings of Mrs. MB were pivotal in the diagnosis of TD. Although the increase in parietal sulci and dilation of the posterior ventricles are frequently observed in the context of normal aging, they are also linked to PCA, which is marked by disproportionate atrophy of the posterior regions of the brain, resulting in prominent initial symptoms, such as visuospatial and topographical deficits, often accompanied by relatively preserved memory in the early phases.
The identification of vascular lacunae indicates a certain level of small vessel disease, which may contribute to cognitive decline and potentially worsen spatial disorientation. The observation of normal hippocampal volumes is particularly significant because hippocampal atrophy is a defining feature of typical amnestic AD. This finding, along with the predominance of her tremor-dominant symptoms over her mild, non-progressive memory fixation issues, suggests that a diagnosis of a non-amnestic variant of AD, including PCA, is more probable than that of typical AD. It also introduces the possibility of mixed pathology, in which both neurodegenerative and vascular elements play a role in the clinical presentation. Additional clinical and neuropsychological assessments are required to establish a definitive diagnosis and exclude other potential causes of TD [12].
The TD is more severe than the typical forgetfulness associated with aging, such as forgetting the parking place of one’s car. Unlike the gradual cognitive decline often seen with age, which may involve slower processing speeds or minor issues with recalling details, topographical memory disorder can lead to a significant inability to orient oneself even in well-known environments [9]. Neuropsychological assessments in these cases frequently highlight specific challenges in spatial memory tasks, mental rotation, and route learning, while other cognitive areas, including language and factual memory, may remain largely unaffected. This distinction, where spatial skills are disproportionately impaired compared to other cognitive abilities, is vital for differentiating this disorder from the general cognitive decline associated with aging or early-stage dementia [11].
Although TD can result from various neurological injuries, such as stroke or traumatic brain injury, its manifestation without significant cognitive impairments may serve as an early sign of AD [8]. In particular, the initial involvement of the hippocampus and entorhinal cortex in the pathology of AD interferes with the formation and retrieval of spatial memories, which are essential for topographical orientation. This issue contrasts with other disorders, such as frontotemporal dementia, where spatial disorientation is less evident in the early phases and is typically associated with pronounced alterations in personality and behavior. Moreover, while other neurological disorders can also affect spatial cognition, the pattern of topographical memory impairment in AD is typically marked by a specific difficulty in recalling routes and identifying landmarks, even when other cognitive abilities, such as language, remain relatively preserved [8].
Numerous individual case studies have offered significant insights into spatial agnosia, topographical memory disorders, and disorientation. One particular case study detailed a patient suffering from severe spatial disorientation who exhibited intact perceptual processing of visual and spatial information. However, this individual displayed a notable dissociation between preserved visual processing and impaired spatial processing during imagery tasks, underscoring the functional independence of these processes and their dependence on distinct neural systems [13]. Another study focused on a patient with visual associative agnosia, who maintained well-functioning language, spatial, visual, and perceptual abilities but struggled to recognize visually presented common objects. This case underscored the essential role of the left hemisphere in the recognition of object meanings [11].
Generally speaking, individuals experiencing TD often find it challenging to reconstruct extensive spatial frameworks and to merge new egocentric and allocentric viewpoints, both of which are essential for effective navigation and successful execution of blind map tasks. Research indicates that the capacity to visualize space in an allocentric manner mentally tends to diminish more significantly with age than the ability to do so egocentrically, which may adversely impact performance in blind map tasks [14].
The evaluation of TD typically relies on clinical and empirical assessments, which offer the advantage of being tailored to the individual and revealing the strategies employed by the patient due to their flexible and non-standardized nature [15, 16]. Such assessments often include tasks such as placing landmarks on a map of a city or country and indicating directions to various locations from the current position [9]. However, the assessment of TD encounters several challenges, as both clinical and spatial-visual tests appear inadequate for accurately identifying cognitive deficits and predicting functional impairments in daily life. This inadequacy is attributed mainly to the absence of a theoretical consensus regarding navigation processes and their underlying neural mechanisms.
To gain deeper insight into the complex relationship between Mrs. MB's neuroanatomical alterations and her cognitive impairments, future studies should utilize specific neuroimaging techniques [17]. Functional MRI (fMRI) can be used to monitor brain activity as patients with TD engage in specific navigation tasks, such as mentally traversing a familiar path or navigating a virtual maze. This approach would help pinpoint the brain areas, particularly those within the parietal and medial temporal lobes, that exhibit reduced activation during these activities. Moreover, Diffusion Tensor Imaging (DTI) [17] can be applied to evaluate the integrity of white matter pathways that connect the hippocampus, entorhinal cortex, and parietal regions. The investigation of these structural links would shed light on whether cognitive deficits stem from a direct lesion or a disruption in the communication pathways among these vital spatial processing centers. Lastly, Positron Emission Tomography (PET) imaging utilizing amyloid or tau tracers could conclusively ascertain whether Alzheimer's pathology is the fundamental cause of the noted neuroanatomical changes, which would be pivotal for validating the diagnosis of AD.
Coupling experimental data with neuroimaging may help decipher the underpinnings of TD. A promising approach entails the use of virtual reality (VR) [18] navigation tasks, which enable researchers to manipulate environmental cues accurately. Participants may be situated within a virtual maze characterized by differing degrees of landmark prominence and path intricacy, while their brain activity is observed through fMRI or EEG. This setup facilitates the exploration of how individuals experiencing TD rely on specific strategies, such as employing egocentric (body-centered) navigation versus allocentric (map-like) navigation, and aids in identifying the neural circuits responsible for these impairments.
Conclusion
The case of Mrs. MB exemplifies the common challenges associated with diagnosing topographical orientation disorder and its impact on a person's navigation abilities. The neuropsychological profile revealed a striking dissociation: while her global cognitive function and executive abilities were preserved mainly, she showed significant impairments in visual object and spatial perception tasks. This specific deficit directly correlated with her reported inability to navigate familiar environments and her poor performance on the blind map task, which assesses allocentric spatial memory. The MRI findings of increased parietal sulci and normal hippocampal volumes are crucial, suggesting a distinct neuroanatomical pattern that is not typical of AD.
These findings underscore that TD is a distinct spatial memory disorder, separate from general age-related cognitive decline. This case illustrates the importance of a targeted neuropsychological evaluation that incorporates specific spatial cognition tasks to distinguish TD from other cognitive impairments. It is crucial to conduct further neuroimaging investigations to clarify the intricate relationships among these functions using fMRI and DTI. This approach would strengthen the diagnostic process and pave the way for more effective, targeted interventions for individuals experiencing TD.
Ethical Considerations
This study does not possess an IRB; nonetheless, all ethical considerations were taken into account in the preparation of this article. The participant was informed about the research's objectives and its diagnostic phases, and she was assured of the confidentiality of her personal information. Additionally, she was given the option to withdraw from the study at any time she desired.
Acknowledgments
The author thanks the patient. He is grateful for editorial staff as well as our university Euromed for their support.
Authors' Contributions
MT conceptualized, wrote and revised the manuscript. He also assessed the patient.
Funding/Support
None.
Conflicts of Interest
The author declares no conflict of interest.
References
Given the aforementioned results, it is clear that Mrs MB displays a specific TD. In addition to the medial temporal lobe, TD may indicate atrophy in the parietal regions, which are essential for spatial processing. Such atrophy in these parietal areas can hinder the integration of sensory information and the creation of mental spatial representations, leading to challenges in navigation, performing blind map tasks, and allocentric spatial memory [10]. These tasks require individuals to place landmarks on a map without visual aids, depending solely on their mental constructs of spatial relationships [11].
The neurocognitive profile displayed by Mrs. MB highlights that her particular TD extends beyond mere age-related forgetfulness, as it encompasses a profound inability to orient herself, even within familiar settings. This observation aligns with the trend of cognitive decline, where spatial abilities are disproportionately affected compared to other cognitive functions, which is essential for differentiating it from general cognitive deterioration or other types of dementia. Although TD may arise from various neurological injuries, such as stroke or traumatic brain injury, its occurrence without notable cognitive deficits can act as an early indicator of AD or an atypical visual variant of AD, including PCA [12].
The MRI findings of Mrs. MB were pivotal in the diagnosis of TD. Although the increase in parietal sulci and dilation of the posterior ventricles are frequently observed in the context of normal aging, they are also linked to PCA, which is marked by disproportionate atrophy of the posterior regions of the brain, resulting in prominent initial symptoms, such as visuospatial and topographical deficits, often accompanied by relatively preserved memory in the early phases.
The identification of vascular lacunae indicates a certain level of small vessel disease, which may contribute to cognitive decline and potentially worsen spatial disorientation. The observation of normal hippocampal volumes is particularly significant because hippocampal atrophy is a defining feature of typical amnestic AD. This finding, along with the predominance of her tremor-dominant symptoms over her mild, non-progressive memory fixation issues, suggests that a diagnosis of a non-amnestic variant of AD, including PCA, is more probable than that of typical AD. It also introduces the possibility of mixed pathology, in which both neurodegenerative and vascular elements play a role in the clinical presentation. Additional clinical and neuropsychological assessments are required to establish a definitive diagnosis and exclude other potential causes of TD [12].
The TD is more severe than the typical forgetfulness associated with aging, such as forgetting the parking place of one’s car. Unlike the gradual cognitive decline often seen with age, which may involve slower processing speeds or minor issues with recalling details, topographical memory disorder can lead to a significant inability to orient oneself even in well-known environments [9]. Neuropsychological assessments in these cases frequently highlight specific challenges in spatial memory tasks, mental rotation, and route learning, while other cognitive areas, including language and factual memory, may remain largely unaffected. This distinction, where spatial skills are disproportionately impaired compared to other cognitive abilities, is vital for differentiating this disorder from the general cognitive decline associated with aging or early-stage dementia [11].
Although TD can result from various neurological injuries, such as stroke or traumatic brain injury, its manifestation without significant cognitive impairments may serve as an early sign of AD [8]. In particular, the initial involvement of the hippocampus and entorhinal cortex in the pathology of AD interferes with the formation and retrieval of spatial memories, which are essential for topographical orientation. This issue contrasts with other disorders, such as frontotemporal dementia, where spatial disorientation is less evident in the early phases and is typically associated with pronounced alterations in personality and behavior. Moreover, while other neurological disorders can also affect spatial cognition, the pattern of topographical memory impairment in AD is typically marked by a specific difficulty in recalling routes and identifying landmarks, even when other cognitive abilities, such as language, remain relatively preserved [8].
Numerous individual case studies have offered significant insights into spatial agnosia, topographical memory disorders, and disorientation. One particular case study detailed a patient suffering from severe spatial disorientation who exhibited intact perceptual processing of visual and spatial information. However, this individual displayed a notable dissociation between preserved visual processing and impaired spatial processing during imagery tasks, underscoring the functional independence of these processes and their dependence on distinct neural systems [13]. Another study focused on a patient with visual associative agnosia, who maintained well-functioning language, spatial, visual, and perceptual abilities but struggled to recognize visually presented common objects. This case underscored the essential role of the left hemisphere in the recognition of object meanings [11].
Generally speaking, individuals experiencing TD often find it challenging to reconstruct extensive spatial frameworks and to merge new egocentric and allocentric viewpoints, both of which are essential for effective navigation and successful execution of blind map tasks. Research indicates that the capacity to visualize space in an allocentric manner mentally tends to diminish more significantly with age than the ability to do so egocentrically, which may adversely impact performance in blind map tasks [14].
The evaluation of TD typically relies on clinical and empirical assessments, which offer the advantage of being tailored to the individual and revealing the strategies employed by the patient due to their flexible and non-standardized nature [15, 16]. Such assessments often include tasks such as placing landmarks on a map of a city or country and indicating directions to various locations from the current position [9]. However, the assessment of TD encounters several challenges, as both clinical and spatial-visual tests appear inadequate for accurately identifying cognitive deficits and predicting functional impairments in daily life. This inadequacy is attributed mainly to the absence of a theoretical consensus regarding navigation processes and their underlying neural mechanisms.
To gain deeper insight into the complex relationship between Mrs. MB's neuroanatomical alterations and her cognitive impairments, future studies should utilize specific neuroimaging techniques [17]. Functional MRI (fMRI) can be used to monitor brain activity as patients with TD engage in specific navigation tasks, such as mentally traversing a familiar path or navigating a virtual maze. This approach would help pinpoint the brain areas, particularly those within the parietal and medial temporal lobes, that exhibit reduced activation during these activities. Moreover, Diffusion Tensor Imaging (DTI) [17] can be applied to evaluate the integrity of white matter pathways that connect the hippocampus, entorhinal cortex, and parietal regions. The investigation of these structural links would shed light on whether cognitive deficits stem from a direct lesion or a disruption in the communication pathways among these vital spatial processing centers. Lastly, Positron Emission Tomography (PET) imaging utilizing amyloid or tau tracers could conclusively ascertain whether Alzheimer's pathology is the fundamental cause of the noted neuroanatomical changes, which would be pivotal for validating the diagnosis of AD.
Coupling experimental data with neuroimaging may help decipher the underpinnings of TD. A promising approach entails the use of virtual reality (VR) [18] navigation tasks, which enable researchers to manipulate environmental cues accurately. Participants may be situated within a virtual maze characterized by differing degrees of landmark prominence and path intricacy, while their brain activity is observed through fMRI or EEG. This setup facilitates the exploration of how individuals experiencing TD rely on specific strategies, such as employing egocentric (body-centered) navigation versus allocentric (map-like) navigation, and aids in identifying the neural circuits responsible for these impairments.
Conclusion
The case of Mrs. MB exemplifies the common challenges associated with diagnosing topographical orientation disorder and its impact on a person's navigation abilities. The neuropsychological profile revealed a striking dissociation: while her global cognitive function and executive abilities were preserved mainly, she showed significant impairments in visual object and spatial perception tasks. This specific deficit directly correlated with her reported inability to navigate familiar environments and her poor performance on the blind map task, which assesses allocentric spatial memory. The MRI findings of increased parietal sulci and normal hippocampal volumes are crucial, suggesting a distinct neuroanatomical pattern that is not typical of AD.
These findings underscore that TD is a distinct spatial memory disorder, separate from general age-related cognitive decline. This case illustrates the importance of a targeted neuropsychological evaluation that incorporates specific spatial cognition tasks to distinguish TD from other cognitive impairments. It is crucial to conduct further neuroimaging investigations to clarify the intricate relationships among these functions using fMRI and DTI. This approach would strengthen the diagnostic process and pave the way for more effective, targeted interventions for individuals experiencing TD.
Ethical Considerations
This study does not possess an IRB; nonetheless, all ethical considerations were taken into account in the preparation of this article. The participant was informed about the research's objectives and its diagnostic phases, and she was assured of the confidentiality of her personal information. Additionally, she was given the option to withdraw from the study at any time she desired.
Acknowledgments
The author thanks the patient. He is grateful for editorial staff as well as our university Euromed for their support.
Authors' Contributions
MT conceptualized, wrote and revised the manuscript. He also assessed the patient.
Funding/Support
None.
Conflicts of Interest
The author declares no conflict of interest.
References
- Denis M. Space and spatial cognition: A multidisciplinary perspective. Routledge; 2017. [Link]
- Epstein RA, Patai EZ, Julian JB, Spiers HJ. The cognitive map in humans: spatial navigation and beyond. Nat Neurosci. 2017;20(11):1504-13. [DOI: 10.1038/nn.4656]
- Huang J, Yang HY, Ruan XG, Yu NG, Zuo GY, Liu HM. A spatial cognitive model that integrates the effects of endogenous and exogenous information on the hippocampus and striatum. Int J Automation Computing. 2021;18:632-44. [DOI:10.1007/s11633-021-1286-z]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci U S A. 2000;97(8):4398-403. [DOI: 10.1073/pnas.070039597] [PMID] [PMCID]
- Maguire EA, Woollett K, Spiers HJ. London taxi drivers and bus drivers: a structural MRI and neuropsychological analysis. Hippocampus. 2006;16(12):1091-101. [DOI: 10.1002/hipo.20233] [PMID]
- Cona G, Scarpazza C. Where is the “where” in the brain? A meta‐analysis of neuroimaging studies on spatial cognition. Hum Brain Mapp. 2019;40(6):1867-86. [DOI: 10.1002/hbm.24496] [PMID]
- Shelton AL, Yamamoto N. Visual memory, spatial representation, and navigation. In Book : Current Issues in Memory. Routledge ; 2021 :27-63. [Link]
- Salmon DP. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. In: Pardon MC, Bondi M. (eds) Behavioral Neurobiology of Aging. 2011:187-212. [DOI :10.1007/7854_2011_171]
- Aguirre GK, D'Esposito M. Topographical disorientation: a synthesis and taxonomy. Brain. 1999;122(9):1613-28. [DOI: 10.1093/brain/122.9.1613]
- Kawakami N, Okada Y, Morihara K, Katsuse K, Kakinuma K, Matsubara S, et al. Long-lasting pure topographical disorientation due to heading disorientation following left retrosplenial infarction: A report of two cases. Brain Cogn. 2024 ;181:106211. [DOI: 10.1016/j.bandc.2024.106211] [PMID]
- Lopez A, Caffò AO, Bosco A. Topographical disorientation in aging. Familiarity with the environment does matter. Neurol Sci. 2018;39(9):1519-28. [DOI: 10.1007/s10072-018-3464-5] [PMID]
- Maia da Silva MN, Millington RS, Bridge H, James-Galton M, Plant GT. Visual dysfunction in posterior cortical atrophy. Front nneurol. 2017;8:389. [DOI: 10.3389/fneur.2017.00389] [PMID] [PMCID]
- Luzzatti C, Vecchi T, Agazzi D, Cesa-Bianchi M, Vergani C. A neurological dissociation between preserved visual and impaired spatial processing in mental imagery. Cortex. 1998;34(3):461-9. [DOI: 10.1016/s0010-9452(08)70768-8] [PMID]
- Mccarthy RA, Warrington EK. Visual associative agnosia: a clinico-anatomical study of a single case. J Neurol Neurosurg Psychiatry. 1986;49(11):1233-40. [DOI: 10.1136/jnnp.49.11.1233] [PMID] [PMCID]
- Taiebine M. Neurocognitive analysis of topographical memory disorder in Alzheimer’s disease from fictional autobiography. Alzheimer's Dementia. 2023;19:e072789. [DOI:10.1002/alz.072789]
- Taiebine M. Neurocognitive stimulation of moroccan bilingual patient with alzheimer’s disease: a case study. Avicenna J Neuro Psycho Physiology. 2024;11(1):7-11. [DOI:10.32592/ajnpp.2024.11.1.101]
- Pengas G, Williams GB, Acosta-Cabronero J, Ash TW, Hong YT, Izquierdo-Garcia D, et al. The relationship of topographical memory performance to regional neurodegeneration in Alzheimer's disease. Fronti Aging Neurosci. 2012;4:17. [DOI: 10.3389/fnagi.2012.00017] [PMID] [PMCID]
- Kober SE, Wood G, Hofer D, Kreuzig W, Kiefer M, Neuper C. Virtual reality in neurologic rehabilitation of spatial disorientation. J Neuroeng Rehabil. 2013;10(1):17. [DOI: 10.1186/1743-0003-10-17 ] [PMID]
Article Type: Case Report |
Subject:
Learning and Memory, Dementia, Alzheimer
Received: 2025/05/13 | Accepted: 2025/03/15 | Published: 2025/06/20
Received: 2025/05/13 | Accepted: 2025/03/15 | Published: 2025/06/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







