Volume 8, Issue 3 (August 2021)
Avicenna J Neuro Psycho Physiology 2021, 8(3): 135-139 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shahbazi A, Azadian E, Zarrinkalam E. Effect of Visual Experience on Spatial Learning of Rats. Avicenna J Neuro Psycho Physiology 2021; 8 (3) :135-139
URL: http://ajnpp.umsha.ac.ir/article-1-300-en.html
URL: http://ajnpp.umsha.ac.ir/article-1-300-en.html
1- M.Sc. Student of Motor Behavior, Department of Motor Behavior, Faculty of Humanities, Islamic Azad University, Hamedan Branch, Hamedan, Iran
2- Assistant Professor, Department of Motor Behavior, Faculty of Humanities, Islamic Azad University, Hamedan Branch, Hamedan, Iran ,azadian1@yahoo.com
3- Assistant Professor, Department of Physical Education and Sport Sciences, Faculty of Humanities, Islamic Azad University, Hamedan Branch, Hamedan, Iran
2- Assistant Professor, Department of Motor Behavior, Faculty of Humanities, Islamic Azad University, Hamedan Branch, Hamedan, Iran ,
3- Assistant Professor, Department of Physical Education and Sport Sciences, Faculty of Humanities, Islamic Azad University, Hamedan Branch, Hamedan, Iran
Full-Text [PDF 719 kb]
(866 Downloads)
| Abstract (HTML) (2879 Views)
start stem (35 cm in length × 10 cm in width × 12 cm in height) of the maze and extended to the intersection of two (L and R) goal arms (70.5 cm in length × 10 cm in width × 12 cm in height). At the end of each goal arm, a plastic cap contained a food reward.
Statistical Analysis
Statistical analyses were conducted in SPSS software (version 16.0). The ANOVA was performed with the post-hoc Tukey test to compare the three groups in terms of the time and number of correct arm entries.
Results
Figure 2 shows the time (S) and the number of correct target box entries during nine days of training. The results of comparing the groups in terms of the number of correct entries showed that there was a significant difference among the three groups regarding the number of correct arm entries in one day (F=6.12, P=0.02). Pairwise post-hoc testing showed that these differences were significant in groups I and III (P=0.3). In other words, group III had fewer correct entries than the other groups. On the third day, there was a significant difference between groups II and III (F=4.53, P=0.04); accordingly, group III had fewer correct entries than group II.
On day seven, group I had a significant difference with groups II and III (F=4.75, p=0.03). Accordingly, group I had fewer correct entries than groups II and III. However, on the other days, there was no significant difference regarding this variable. At the same time, there was a significant difference between the three groups in terms of the mean of correct arm entries (F=4.93, P=0.036). Comparison of the results revealed that the number
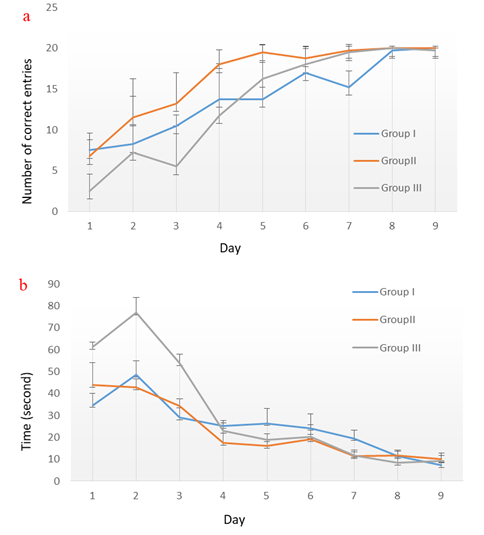
Figure 2. a) Number and b) time of correct target box entries during nine days of training
Full-Text: (970 Views)
Background
Vision plays a very important role in exploring and navigating the environment. Visual information acquired through observation plays a pivotal role in learning a movement pattern and motor control [1]. In the early stages of evolution, synaptic connections experience major changes caused by the sensory experience; therefore, any change in the visual information will have profound effects on the performance of neural circuits in the visual cortex [2].
Currently, over 61,000 children suffer from visual impairment. Studies have shown that these children fall behind the children with normal eyesight in terms of physical and motor activities [3,4]. Problems in learning the environment decrease the direct and complete environmental perception in blind individuals to some extent [5]. According to Nakamura [6], blind people walk slower than their sighted counterparts since they adopt precautionary measures to avoid collisions or falls. However, based on the findings of a study, the walking speed of the sighted participants with their eyes closed (given their visual experience of the laboratory) was significantly lower than the walking speed of the blind subjects [7].
Furthermore, the congenitally blind participants had less spatial awareness than the sighted and the lately blinded participants [8]. However, there are also studies and evidence indicating the equal potential of the blind in delivering motor performance, compared to the individuals with normal eyesight, especially when they are offered equal opportunities and encouragements. For instance, in the 2016 Summer Paralympics (Rio, Brazil), four visually-impaired runners outperformed the sighted runners in the 1,500-meter race [5,8]. There is a considerable amount of literature on the importance of visual information in movement control [9-13]; nevertheless, only a few researchers have addressed motor learning in blind individuals.
According to the bulk of studies on the effects of vision on learning and memory among rats, there is a direct relationship between accurate spatial navigation and visual performance in animals. Finding food successfully, reproducing, evading predators and hunters, defending habitats, and locating positions accurately depend on visual information [14]. Based on the findings of a study on light deprivation, rats that were grown in total darkness outperformed the control group (receiving natural light) in the water maze (navigation task) [15]. Other studies have pointed to the hindered performance of animals caused by growing in total darkness [16].
Objectives
Based on these contradictory results, this study aimed to analyze the effect of a visual deprivation period on the spatial learning of rats.
Materials and Methods
Animals and Preparations
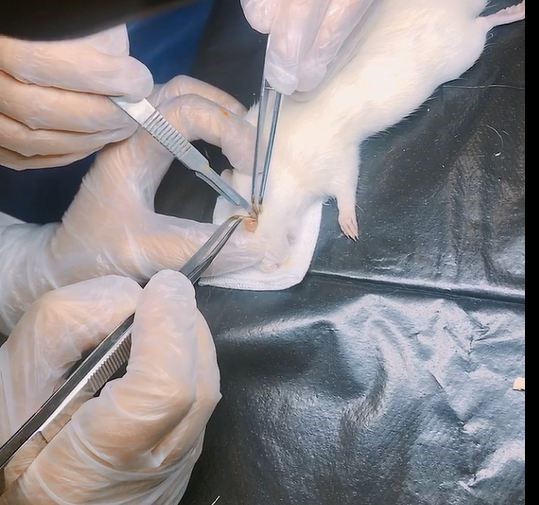
In total, 12 male rats were randomly divided into three equal groups (group I, II, and III). It should be mentioned that the four rats in group I were used as the control group [17]. Transection of the two optic nerves was performed on group II on the seventh day after birth and group III on the seventh week after birth to develop the early blinded and lately blinded models, respectively. The study was conducted when the rats were eight weeks old. The optic nerve transection protocol was as follows: the tissues around the eyeball were bluntly transected and dissected under an operating microscope until the optic nerve was clearly exposed. Next, a small section of the optic nerve was removed from the surgical eye to ensure that the optic nerve was completely transected (Figure 1) [18].
Afterward, a T-shaped maze task was conducted based on absolute orientation for which the rats were trained to turn right at the T-junction. The rats were placed in a start box for 30 sec and after that were allowed to freely explore the T-shaped maze for up to 120 sec until they found the pellets. During the entire session, the T-shaped maze was placed at the same location in the same testing room. It should be mentioned that 20 trials per day were conducted for nine consecutive days [5] The time and number of correct arm entries were recorded.
Apparatus
The behavioral apparatus was a black wooden T- maze. A start box (10 cm in length) opened to the
Currently, over 61,000 children suffer from visual impairment. Studies have shown that these children fall behind the children with normal eyesight in terms of physical and motor activities [3,4]. Problems in learning the environment decrease the direct and complete environmental perception in blind individuals to some extent [5]. According to Nakamura [6], blind people walk slower than their sighted counterparts since they adopt precautionary measures to avoid collisions or falls. However, based on the findings of a study, the walking speed of the sighted participants with their eyes closed (given their visual experience of the laboratory) was significantly lower than the walking speed of the blind subjects [7].
Furthermore, the congenitally blind participants had less spatial awareness than the sighted and the lately blinded participants [8]. However, there are also studies and evidence indicating the equal potential of the blind in delivering motor performance, compared to the individuals with normal eyesight, especially when they are offered equal opportunities and encouragements. For instance, in the 2016 Summer Paralympics (Rio, Brazil), four visually-impaired runners outperformed the sighted runners in the 1,500-meter race [5,8]. There is a considerable amount of literature on the importance of visual information in movement control [9-13]; nevertheless, only a few researchers have addressed motor learning in blind individuals.
According to the bulk of studies on the effects of vision on learning and memory among rats, there is a direct relationship between accurate spatial navigation and visual performance in animals. Finding food successfully, reproducing, evading predators and hunters, defending habitats, and locating positions accurately depend on visual information [14]. Based on the findings of a study on light deprivation, rats that were grown in total darkness outperformed the control group (receiving natural light) in the water maze (navigation task) [15]. Other studies have pointed to the hindered performance of animals caused by growing in total darkness [16].
Objectives
Based on these contradictory results, this study aimed to analyze the effect of a visual deprivation period on the spatial learning of rats.
Materials and Methods
Animals and Preparations
In total, 12 male rats were randomly divided into three equal groups (group I, II, and III). It should be mentioned that the four rats in group I were used as the control group [17]. Transection of the two optic nerves was performed on group II on the seventh day after birth and group III on the seventh week after birth to develop the early blinded and lately blinded models, respectively. The study was conducted when the rats were eight weeks old. The optic nerve transection protocol was as follows: the tissues around the eyeball were bluntly transected and dissected under an operating microscope until the optic nerve was clearly exposed. Next, a small section of the optic nerve was removed from the surgical eye to ensure that the optic nerve was completely transected (Figure 1) [18].
Afterward, a T-shaped maze task was conducted based on absolute orientation for which the rats were trained to turn right at the T-junction. The rats were placed in a start box for 30 sec and after that were allowed to freely explore the T-shaped maze for up to 120 sec until they found the pellets. During the entire session, the T-shaped maze was placed at the same location in the same testing room. It should be mentioned that 20 trials per day were conducted for nine consecutive days [5] The time and number of correct arm entries were recorded.
Apparatus
The behavioral apparatus was a black wooden T- maze. A start box (10 cm in length) opened to the

Figure 1.Optic nerve transection protocol in rats
start stem (35 cm in length × 10 cm in width × 12 cm in height) of the maze and extended to the intersection of two (L and R) goal arms (70.5 cm in length × 10 cm in width × 12 cm in height). At the end of each goal arm, a plastic cap contained a food reward.
Statistical Analysis
Statistical analyses were conducted in SPSS software (version 16.0). The ANOVA was performed with the post-hoc Tukey test to compare the three groups in terms of the time and number of correct arm entries.
Results
Figure 2 shows the time (S) and the number of correct target box entries during nine days of training. The results of comparing the groups in terms of the number of correct entries showed that there was a significant difference among the three groups regarding the number of correct arm entries in one day (F=6.12, P=0.02). Pairwise post-hoc testing showed that these differences were significant in groups I and III (P=0.3). In other words, group III had fewer correct entries than the other groups. On the third day, there was a significant difference between groups II and III (F=4.53, P=0.04); accordingly, group III had fewer correct entries than group II.
On day seven, group I had a significant difference with groups II and III (F=4.75, p=0.03). Accordingly, group I had fewer correct entries than groups II and III. However, on the other days, there was no significant difference regarding this variable. At the same time, there was a significant difference between the three groups in terms of the mean of correct arm entries (F=4.93, P=0.036). Comparison of the results revealed that the number

Figure 2. a) Number and b) time of correct target box entries during nine days of training
of correct arm entries of group II was greater than that of group III.
Moreover, there was a significant difference among the three groups regarding the time it took the rats to enter the target box on the second (F=9.52, P=0.006) and third days (F=4.23, P=0.047). On the second day, the time it took the rats in group III to enter the target box was significantly longer than that of rats in the other groups (group I: P=0.02, group II: P=0.008). On the third day, there was a difference between groups I and III regarding the time (P=0.049).
Discussion
This study aimed to investigate the effects of a period of visual deprivation on the spatial learning of rats. The results showed that, during the first days, the number of correct goal box entries in group III was lower than that of the other two groups; this difference was significant on days one and three. Analysis of Figure 2 revealed that from day two onwards, more than 50% of the attempts of group II to enter the goal box correctly were successful. Moreover, this was the case with group I on day three and group III from day five onwards.
The total mean number of successful entry attempts in these nine days was found to be significantly higher in groups I and II, compared to group III. This means that the early blinded rats showed greater ability in spatial exploration than rats in group III. Moreover, there was a significant difference in the time of entry only on days two and three. According to the results, on days two and three, it took the rats in group III a longer time to enter the target box, compared to the other two groups. From day five to nine, the difference between the groups started to disappear, and the performance of the three groups finally reached the set upper limit which was 20 successful attempts on day nine.
According to the findings of a study conducted by Norimoto and Ikegaya in 2015 on blind rats, the success rate of entering the goal box remained at 50% after nine consecutive days of practice in the T-maze [5]. Their finding was inconsistent with those of the present study since they changed the orientation of the T-maze randomly for every attempt, whereas the path and goal box did not change in the present study. In the current research, the lately blinded rat (group III) needed longer time to enter the target box and also had fewer correct entries, compared to the other groups. The results of this study are in line with those of previous studies which showed that the sighted rats performed better than blind rats in spatial learning [19-21].
In some previous studies, the researchers have compared the effect of visual experience on spatial learning of rats that were bred in dark and light rooms. Based on the results of these studies, the rats that were bred in light rooms showed better performance in the learning task (water maze). However, rats that were bred in dark rooms observed the environment during the experiments and performed more slowly due to their dependence on non-visual cues [17,19,22].
Richard et al. (1981) found that early visual experience could affect the development of the ability to acquire the spatial concept. The rats that were grown in a dark environment performed less effectively than blind rats and the rats that were grown in a light environment [21]. In order to investigate the effect of visual experience on learning, they used blind rats that had become blind in adulthood, similar to the present study (novelty). However, in the present study rats were grown from infancy to maturity in conditions of absolute blindness (group II). In this regard, results of a study performed by Melzer et al. in 2007) showed that growing up blind has a different effect on CNS (central nervous system) than growing up in a darkroom or becoming blind in adulthood [17].
On the other hand, the result of the present study showed that spatial learning was the same and even better in the early blinded rats (group II) than in the control group. Voller et al. in 2014 investigated the effects of vision and whiskers on coordination of turning and gait parameters. They found that long-term loss of vision and whiskers increased the use of other motor control mechanisms (e.g., muscle tone, coordination of posture, and movement), proprioceptive system, vestibular, and somatosensory systems in the claws which is in line with the results of the present study. Voller et al. concluded that whiskers compensated for the loss of vision, especially in activities that required the coordination and maintenance of posture. Moreover, based on their results, there was a significant difference between blind and sighted rats in unconscious movements [23]. These findings indicate that rats with longer visual deprivation experienced changes in CNS, which boosted sensitivity in their hearing and other proprioceptive senses. Therefore, it can be said that whiskers significantly enhance the performance of rats [24,25].
One of the most important findings of the present study was that having visual experience in performing routine tasks could not be effective in performing a new task. As a result, the lately blinded rats that had not yet developed brain adaptation, had low speed and accuracy in learning new tasks, compared to group III. This might be due to the dependence of rats on visual information and their lack of use of other information collected through other senses.
The spatial learning rate was the same in early blinded rats as those with normal eyesight which is due to their use of other senses to compensate for the lack of visual information [26]. There are also other contributing factors, including the information received by tactile receptors [27] and muscles about shape, texture, and stiffness [28] as well as the neuro-plastic adaptation that occurs throughout the entire cerebral cortex [29].
Conclusions
Comparison of the findings of this study with those of previous studies led to the conclusion that visual deprivation affected spatial learning and that rats that had lost their vision during infancy showed an equal or even better performance than the sighted rats during the early days of the experiment. However, over time (nine days), there was an increase in the rate of their learning, including the speed and the number of entries to the goal box. In conclusion, the period of visual deprivation was an important factor in motor learning and performance. However, having visual experience in performing routine tasks could not be helpful in performing a new task.
Compliance with ethical guidelines
Ethical Approval
This study was approved by the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (code: IR.UMSHA.REC.1398.257).
Conflicts of Interest
The authors declare no potential conflicts of interest relevant to this article.
Acknowledgments
The authors would like to appreciate the officials of the Animal Laboratory of Hamadan University of Medical Sciences and the Islamic Azad University of Hamadan, Hamadan, Iran.
Funding/Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
Moreover, there was a significant difference among the three groups regarding the time it took the rats to enter the target box on the second (F=9.52, P=0.006) and third days (F=4.23, P=0.047). On the second day, the time it took the rats in group III to enter the target box was significantly longer than that of rats in the other groups (group I: P=0.02, group II: P=0.008). On the third day, there was a difference between groups I and III regarding the time (P=0.049).
Discussion
This study aimed to investigate the effects of a period of visual deprivation on the spatial learning of rats. The results showed that, during the first days, the number of correct goal box entries in group III was lower than that of the other two groups; this difference was significant on days one and three. Analysis of Figure 2 revealed that from day two onwards, more than 50% of the attempts of group II to enter the goal box correctly were successful. Moreover, this was the case with group I on day three and group III from day five onwards.
The total mean number of successful entry attempts in these nine days was found to be significantly higher in groups I and II, compared to group III. This means that the early blinded rats showed greater ability in spatial exploration than rats in group III. Moreover, there was a significant difference in the time of entry only on days two and three. According to the results, on days two and three, it took the rats in group III a longer time to enter the target box, compared to the other two groups. From day five to nine, the difference between the groups started to disappear, and the performance of the three groups finally reached the set upper limit which was 20 successful attempts on day nine.
According to the findings of a study conducted by Norimoto and Ikegaya in 2015 on blind rats, the success rate of entering the goal box remained at 50% after nine consecutive days of practice in the T-maze [5]. Their finding was inconsistent with those of the present study since they changed the orientation of the T-maze randomly for every attempt, whereas the path and goal box did not change in the present study. In the current research, the lately blinded rat (group III) needed longer time to enter the target box and also had fewer correct entries, compared to the other groups. The results of this study are in line with those of previous studies which showed that the sighted rats performed better than blind rats in spatial learning [19-21].
In some previous studies, the researchers have compared the effect of visual experience on spatial learning of rats that were bred in dark and light rooms. Based on the results of these studies, the rats that were bred in light rooms showed better performance in the learning task (water maze). However, rats that were bred in dark rooms observed the environment during the experiments and performed more slowly due to their dependence on non-visual cues [17,19,22].
Richard et al. (1981) found that early visual experience could affect the development of the ability to acquire the spatial concept. The rats that were grown in a dark environment performed less effectively than blind rats and the rats that were grown in a light environment [21]. In order to investigate the effect of visual experience on learning, they used blind rats that had become blind in adulthood, similar to the present study (novelty). However, in the present study rats were grown from infancy to maturity in conditions of absolute blindness (group II). In this regard, results of a study performed by Melzer et al. in 2007) showed that growing up blind has a different effect on CNS (central nervous system) than growing up in a darkroom or becoming blind in adulthood [17].
On the other hand, the result of the present study showed that spatial learning was the same and even better in the early blinded rats (group II) than in the control group. Voller et al. in 2014 investigated the effects of vision and whiskers on coordination of turning and gait parameters. They found that long-term loss of vision and whiskers increased the use of other motor control mechanisms (e.g., muscle tone, coordination of posture, and movement), proprioceptive system, vestibular, and somatosensory systems in the claws which is in line with the results of the present study. Voller et al. concluded that whiskers compensated for the loss of vision, especially in activities that required the coordination and maintenance of posture. Moreover, based on their results, there was a significant difference between blind and sighted rats in unconscious movements [23]. These findings indicate that rats with longer visual deprivation experienced changes in CNS, which boosted sensitivity in their hearing and other proprioceptive senses. Therefore, it can be said that whiskers significantly enhance the performance of rats [24,25].
One of the most important findings of the present study was that having visual experience in performing routine tasks could not be effective in performing a new task. As a result, the lately blinded rats that had not yet developed brain adaptation, had low speed and accuracy in learning new tasks, compared to group III. This might be due to the dependence of rats on visual information and their lack of use of other information collected through other senses.
The spatial learning rate was the same in early blinded rats as those with normal eyesight which is due to their use of other senses to compensate for the lack of visual information [26]. There are also other contributing factors, including the information received by tactile receptors [27] and muscles about shape, texture, and stiffness [28] as well as the neuro-plastic adaptation that occurs throughout the entire cerebral cortex [29].
Conclusions
Comparison of the findings of this study with those of previous studies led to the conclusion that visual deprivation affected spatial learning and that rats that had lost their vision during infancy showed an equal or even better performance than the sighted rats during the early days of the experiment. However, over time (nine days), there was an increase in the rate of their learning, including the speed and the number of entries to the goal box. In conclusion, the period of visual deprivation was an important factor in motor learning and performance. However, having visual experience in performing routine tasks could not be helpful in performing a new task.
Compliance with ethical guidelines
Ethical Approval
This study was approved by the Ethics Committee of Hamadan University of Medical Sciences, Hamadan, Iran (code: IR.UMSHA.REC.1398.257).
Conflicts of Interest
The authors declare no potential conflicts of interest relevant to this article.
Acknowledgments
The authors would like to appreciate the officials of the Animal Laboratory of Hamadan University of Medical Sciences and the Islamic Azad University of Hamadan, Hamadan, Iran.
Funding/Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Effenberg AO. Movement sonification: Effects on perception and action. IEEE Multimedia. 2005; 12(2):53-9. [DOI: 10.1109/MMUL.2005.31]
- Crowley JC, Katz LC. Development of ocular dominance columns in the absence of retinal input. Nature Neuroscience. 1999; 2(12):1125-30. [DOI:10.1038/16051] [PMID]
- Augestad LB, Jiang L. Physical activity, physical fitness, and body composition among children and young adults with visual impairments: a systematic review. British Journal of Visual Impairment. 2015; 33(3):167-82. [DOI: 10.1177/0264619615599813]
- Lieberman LJ, Lepore M, Lepore-Stevens M, Ball L. Physical education for children with visual impairment or blindness. Journal of Physical Education, Recreation & Dance. 2019; 90(1):30-8. [DOI:10.1080/07303084.2018.1535340]
- Norimoto H, Ikegaya Y. Visual cortical prosthesis with a geomagnetic compass restores spatial navigation in blind rats. Current Biology. 2015; 25(8):1091-5. [DOI: 10.1016/j.cub.2015.02.063] [PMID]
- Nakamura T. Quantitative analysis of gait in the visually impaired. Disability and Rehabilitation. 1997; 19(5):194-7. [DOI:10.3109/09638289709166526] [PMID]
- Hallemans A, Ortibus E, Meire F, Aerts P. Low vision affects dynamic stability of gait. Gait & Posture. 2010; 32(4):547-51. [DOI:10.1016/j.gaitpost.2010.07.018] [PMID]
- Pasqualotto A, Proulx MJ. The role of visual experience for the neural basis of spatial cognition. Neuroscience & Biobehavioral Reviews. 2012; 36(4):1179-87. [DOI: 10.1016/j.neubiorev.2012.01.008] [PMID]
- Hallemans A, Ortibus E, Truijen S, Meire F. Development of independent locomotion in children with a severe visual impairment. Research in Developmental Disabilities. 2011; 32(6):2069-74. [DOI:10.1016/j.ridd.2011.08.017] [PMID]
- Carlton LG. Processing visual feedback information for movement control. Journal of Experimental Psychology: Human Perception and Performance. 1981; 7(5):1019-30. [DOI:10.1037/0096-1523.7.5.1019] [PMID]
- Lim YH, Lee HC, Falkmer T, Allison GT, Tan T, Lee WL, et al. Effect of visual information on postural control in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2019; 49(12):4731-9. [DOI: 10.1007/s10803-018-3634-6] [PMID]
- Kuiper OX, Bos JE, Diels C, Schmidt EA. Knowing what's coming: anticipatory audio cues can mitigate motion sickness. Applied Ergonomics. 2020; 85:103068. [DOI:10.1016/j.apergo.2020.103068] [PMID]
- Pechtl KS, Jennings JR, Redfern MS. Optic flow and attention alter locomotion differently in the young and old. Gait & Posture. 2020; 76:1-6. [DOI:10.1016/j.gaitpost.2019.10.022] [PMID]
- Akesson S, Helm B. Endogenous programs and flexibility in bird migration. Frontiers in Ecology and Evolution. 2020; 8:78. [DOI: 10.3389/fevo.2020.00078]
- Salami M. Light deprivation-related changes of strategy selection in the radial arm maze. Physiological Research. 2007; 56(1):123-8. [PMID]
- Chow KL, Spear PD. Morphological and functional effects of visual deprivation on the rabbit visual system. Experimental Neurology. 1974; 42(2):429-47. [DOI:10.1016/0014-4886(74)90036-3] [PMID]
- Melzer P, Mineo L, Ebner FF. Optic nerve transection affects development and use-dependent plasticity in neocortex of the rat: Quantitative acetylcholinesterase imaging. Brain Research. 2007; 1139:68-84. [DOI:10.1016/j.brainres.2006.12.080] [PMID]
- Wang R, Zhong Y, Tang W, Tang Z, Sun X, Feng X, et al. Evaluation of changes in magnetic resonance diffusion tensor imaging of the bilateral optic tract in monocular blind rats. International Journal of Developmental Neuroscience. 2017; 59:10-4. [DOI:10.1016/j.ijdevneu.2017.02.006] [PMID]
- Tees RC, Buhrmann K, Hanley J. The effect of early experience on water maze spatial learning and memory in rats. Developmental Psychobiology. 1990; 23(5):427-39. [DOI:10.1002/dev.420230505] [PMID]
- Zavareh SA, Davari S, Gholami M, Salami M. Change in visual experience impairs rat's spatial learning in morris water maze. Journal of Isfahan Medical School. 2010; 28(111):1-12.
- Tees RC, Midgley G, Nesbit JC. The effect of early visual experience on spatial maze learning in rats. Developmental Psychobiology. 1981; 14(5):425-38. [DOI:10.1002/dev.420140505] [PMID]
- Rose S. Early visual experience, learning, and neurochemical plasticity in the rat and the chick. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 1977; 278(961):307-18. [DOI: 10.1098/rstb.1977.0044] [PMID]
- Voller J, Potužáková B, Šimeček V, Vožeh F. The role of whiskers in compensation of visual deficit in a mouse model of retinal degeneration. Neuroscience Letters. 2014; 558:149-53. [DOI:10.1016/j.neulet.2013.11.005] [PMID]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behavioural Brain Research. 2001; 125(1-2):141-9. [DOI:10.1016/s0166-4328(01)00291-1] [PMID]
- Benaroya‐Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. European Journal of Neuroscience. 2004; 20(5):1341-7. [DOI:10.1111/j.1460-9568.2004.03587.x] [PMID]
- Nakata H, Yabe K. Automatic postural response systems in individuals with congenital total blindness. Gait & Posture. 2001; 14(1):36-43. [DOI:10.1016/s0966-6362(00)00100-4] [PMID]
- Leo A, Bernardi G, Handjaras G, Bonino D, Ricciardi E, Pietrini P. Increased BOLD variability in the parietal cortex and enhanced parieto-occipital connectivity during tactile perception in congenitally blind individuals. Neural Plasticity. 2012; 2012:720278. [DOI:10.1155/2012/720278] [PMID] [PMCID]
- Johnson KO, Hsiao SS. Neural mechanisms of tactual form and texture perception. Annual Review of Neuroscience. 1992; 15(1):227-50. [DOI:10.1146/annurev.ne.15.030192.001303] [PMID]
- Lingnau A, Strnad L, He C, Fabbri S, Han Z, Bi Y, et al. Cross-modal plasticity preserves functional specialization in posterior parietal cortex. Cerebral Cortex. 2012; 24(2):541-9. [DOI:10.1093/cercor/bhs340] [PMID].
Article Type: Research Article |
Subject:
Learning and Memory, Dementia, Alzheimer
Received: 2020/08/4 | Accepted: 2020/10/5 | Published: 2021/06/20
Received: 2020/08/4 | Accepted: 2020/10/5 | Published: 2021/06/20
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







