Volume 4, Issue 4 (November 2017)
Avicenna J Neuro Psycho Physiology 2017, 4(4): 153-162 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Salimi Z, Moradpour F, Khajehpour L, Moazedi A A, Pourmotabbed A. The Role of Aromatase and Castration on Spatial Learning and Memory Changes by Nandrolone. Avicenna J Neuro Psycho Physiology 2017; 4 (4) :153-162
URL: http://ajnpp.umsha.ac.ir/article-1-109-en.html
URL: http://ajnpp.umsha.ac.ir/article-1-109-en.html
1- Department of Biology, Faculty of Science, Shahid Chamran University of Ahvaz, Ahvaz, Iran.
2- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. ,fmoradpour@kums.ac.ir
3- Department of Physiology, Faculty of Science, Kermanshah University of Medical Sciences, Kermanshah, Iran.
2- Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran. ,
3- Department of Physiology, Faculty of Science, Kermanshah University of Medical Sciences, Kermanshah, Iran.
Full-Text [PDF 888 kb]
(1107 Downloads)
| Abstract (HTML) (3176 Views)
Full-Text: (1170 Views)
1. Introduction
The abuse of Anabolic Androgenic Steroids (AASs) by athletics for aesthetics purposes have increased among adolescents during recent decades [1]. Adolescents are more susceptible to the deleterious effects of AASs, compared to adults [2]. Nandrolone Decanoate (ND) is one of the most popular AAS compounds that are majorly abused [3]. ND impairs learning and memory in adults [1, 4]; however, the underlying mechanism is unclear. Literature which describes the effects of androgenic compounds on the spatial learning and memory of animals and humans are contradictory. Some evidence has suggested that the intrahippocampal microinjection of testosterone or their metabolites can impair spatial learning and information retention [5, 6]. Contrarily, several studies indicated that chronic treatment with androgens has improved spatial memory [7, 8].
This discrepancy may arise from the point that the intrahippocampal microinjection of androgens has more region-specific effects than systemic treatment. Moreover, the systemic treatment of androgens has a longer half-life than intrahippocampal injection. Therefore, the intracerebroventricular injection of ND could expose all the brain to ND for only a short time. Kouvelas et al. argued that injected ND (with doses 15 mg/ kg subcutaneously, once daily for 6 wk) induced anxiolytic-like behavior, impair social memory via activating central androgen receptors (AR) [4].
The androgenic receptor is not necessarily the only mechanism of action for ND in the Central Nervous System (CNS). ND can also be converted to estrogen through oxidation and the subsequent elimination of methyl groups [3]. Therefore, another mechanism of ND on memory may be mediating estradiol and the effect of aromatase which converts ND to estradiol. However, data about the role of P450 aromatase in the mediation of ND effects on spatial learning and memory are scarce. Therefore, this assumption was investigated by the application of anastrozole as an aromatase inhibitor. Additionally, ND stimulates aromatase activity to the conversion of intrinsic testosterone to estradiol [9, 10].
Previous studies reported that testosterone may change spatial learning through conversion to estradiol by P450 aromatase [6]. This mechanism could partially mediate the effect of ND on spatial learning and memory. Moreover, ND administration increases testosterone plasma level through influencing hypothalamus-hypophysis-gonads axis [11]. The improvement effect of systemic testosterone on spatial learning and memory is well documented [7, 8, 12]. Therefore, ND might affect spatial learning and memory by elevating plasma testosterone level. Consequently, this study evaluated the effect of castration on spatial learning and memory changes induced by ND. Briefly, the current study aimed to evaluate the role of P450 aromatase and castration on spatial learning and memory changes induced by Nandrolone in adolescent male rats.
2. Materials and Methods
Animals
Male Wistar rats aged 25-27 days were initially obtained from the animal house of Shahid Chamran University of Ahvaz. The animals were housed in a plexiglass cage under controlled temperature (22±2°C), with daily exposure to a 12-hour light-dark cycle (lights on at 7:00 AM). All rats had free access to a standard diet and tap water.
Surgery
The rats were anesthetized with a mixture of ketamine and xylazine (100 mg/ kg and 25 mg/ kg, respectively; Intraperitoneal [IP] injection.) and placed in a stereotaxic instrument. A guide cannula was implanted in the right lateral ventricle of all rats. Stereotaxic coordinates based on Paxinos and Watsons atlas of the rat brain were as follows: Anterior-Posterior (AP), -0.9 mm from bregma; Medial-Lateral (ML), -1.2 mm from midline; and Dorsal-Ventral (DV), -3.4 mm from the skull surface [13]. Castration was also performed by the incision of the sac of the scrotum along with underlying tunica. The vas deferens was bilaterally tied off with a sterile chromic ligature, and both testes were removed; thereafter, incisions on the scrotum were sutured. The place of surgery was disinfected using betadine. Furthermore, the topical analgesic (2.5% lidocaine, 2.5% prilocaine) was applied to the incision site immediately after the surgery [14]. All procedures were approved by the local Animal Ethics Committee (EE/ 97, 24, 3061300/ scu.ac.ir).
Microinjection procedure
In this experimental study, male Wistar rats were divided into 10 groups. The experimental groups received DMSO as control groups and the different doses of ND (10, 30 and 60 µg/ 2.5 µL), anastrozole (2.5, 5 and 10 µg/ 2.5 µL), and anastrozole (2.5 µg/ 2.5 µL) + ND (60 µg/ 2.5 µL) all days before the training. The rats of ninth and tenth groups were castrated and treated with 2.5 µL of DMSO and ND (60 µg), respectively for 4 days.
Treatments initiated at 7 days (recovery time) after conducting the surgery. The intracerebral injection was administered through guide cannula (22-gauge) using injection needles (27-gauge) connected by polyethylene tubing to a Hamilton microsyringe. The injection needle was inserted 0.5 mm beyond the tip of the guide cannula. Then, vehicle (dimethyl sulfoxide, DMSO 10%) or different doses of ND and anastrozole were injected into the right lateral ventricle. All injections were performed at 30 min before testing, each day (during acquisition days). The other procedure of injection was administered for rats that were received two adjunct medications. In this procedure, anastrozole plus ND was injected with a 15-min interval. The injections (2.5 µL volume) were performed in 2.5 min and the injection needles were left in place for an additional minute. The drugs first dissolved in pure DMSO; then, the solution was diluted by the addition of saline solution to reach a final drug concentration.
Behavioral study
Spatial learning and memory were investigated using the Morris Water Maze (MWM) test. It consisted of a dark gray circular pool filled with water (24±1◦C) and a transparent platform submerged 1cm below the surface of the water. The pool was located in a room exclusively used for behavioral studies with a constant environment and visual cues on walls around the pool. Each trial was recorded and analyzed by a video camera connected to a tracking system (EthoVision XT) which was mounted above the pool. The test was designed in 4 trials for 4 consecutive days and probe trial was done on the fifth day.
The pool was divided into 4 parts, including north-west, northeast, south-west, and south-east. At the beginning of each trial, an animal was randomly placed facing the wall of the pool at one of 4 different starting points (north, east, south, and west). A maximum time of 60s was allowed to locate the hidden platform, which was situated in the north-west quadrant during all training trials. The rats were gently guided to the platform by the investigator, when not finding the platform within the maximum time. After locating, the platform rats were allowed to remain there for 30s. The platform was subsequently removed during the probe trial (at the fifth day) and the animals were allowed to search the platform location for 60s. The amount of time spent by the rats in target quadrants was determined [15].
Histology
At the end of the behavioral procedures, the injection site was histologically verified. The rats were deeply anesthetized and 2.5 µL of a solution of 4% methylene blue saline was infused into the implanted site; then, they were intracardially perfused with buffer phosphate saline, followed by 2% formalin. The rats’ brains were removed, the required sections were collected and the cannula tracks were examined for each rat. The subjects without adequate histological evidence were excluded from data processing (Figure 1) [16].
Experiments
Experiment 1: The effects of ND on spatial learning and memory in the MWM
The present experiment determined the effects of ND Intracerebroventricular (ICV) microinjection on MWM performance. The studied rats were divided into 4 groups according to the dose levels; 0 (n=11), 10(n=9), 30(n=10), and 60 µg (n=9) of ND dissolved in 2.5 µL of DMSO for 4 learning days.
Experiment 2: The effects of anastrozole on spatial learning and memory in the MWM
The abuse of Anabolic Androgenic Steroids (AASs) by athletics for aesthetics purposes have increased among adolescents during recent decades [1]. Adolescents are more susceptible to the deleterious effects of AASs, compared to adults [2]. Nandrolone Decanoate (ND) is one of the most popular AAS compounds that are majorly abused [3]. ND impairs learning and memory in adults [1, 4]; however, the underlying mechanism is unclear. Literature which describes the effects of androgenic compounds on the spatial learning and memory of animals and humans are contradictory. Some evidence has suggested that the intrahippocampal microinjection of testosterone or their metabolites can impair spatial learning and information retention [5, 6]. Contrarily, several studies indicated that chronic treatment with androgens has improved spatial memory [7, 8].
This discrepancy may arise from the point that the intrahippocampal microinjection of androgens has more region-specific effects than systemic treatment. Moreover, the systemic treatment of androgens has a longer half-life than intrahippocampal injection. Therefore, the intracerebroventricular injection of ND could expose all the brain to ND for only a short time. Kouvelas et al. argued that injected ND (with doses 15 mg/ kg subcutaneously, once daily for 6 wk) induced anxiolytic-like behavior, impair social memory via activating central androgen receptors (AR) [4].
The androgenic receptor is not necessarily the only mechanism of action for ND in the Central Nervous System (CNS). ND can also be converted to estrogen through oxidation and the subsequent elimination of methyl groups [3]. Therefore, another mechanism of ND on memory may be mediating estradiol and the effect of aromatase which converts ND to estradiol. However, data about the role of P450 aromatase in the mediation of ND effects on spatial learning and memory are scarce. Therefore, this assumption was investigated by the application of anastrozole as an aromatase inhibitor. Additionally, ND stimulates aromatase activity to the conversion of intrinsic testosterone to estradiol [9, 10].
Previous studies reported that testosterone may change spatial learning through conversion to estradiol by P450 aromatase [6]. This mechanism could partially mediate the effect of ND on spatial learning and memory. Moreover, ND administration increases testosterone plasma level through influencing hypothalamus-hypophysis-gonads axis [11]. The improvement effect of systemic testosterone on spatial learning and memory is well documented [7, 8, 12]. Therefore, ND might affect spatial learning and memory by elevating plasma testosterone level. Consequently, this study evaluated the effect of castration on spatial learning and memory changes induced by ND. Briefly, the current study aimed to evaluate the role of P450 aromatase and castration on spatial learning and memory changes induced by Nandrolone in adolescent male rats.
2. Materials and Methods
Animals
Male Wistar rats aged 25-27 days were initially obtained from the animal house of Shahid Chamran University of Ahvaz. The animals were housed in a plexiglass cage under controlled temperature (22±2°C), with daily exposure to a 12-hour light-dark cycle (lights on at 7:00 AM). All rats had free access to a standard diet and tap water.
Surgery
The rats were anesthetized with a mixture of ketamine and xylazine (100 mg/ kg and 25 mg/ kg, respectively; Intraperitoneal [IP] injection.) and placed in a stereotaxic instrument. A guide cannula was implanted in the right lateral ventricle of all rats. Stereotaxic coordinates based on Paxinos and Watsons atlas of the rat brain were as follows: Anterior-Posterior (AP), -0.9 mm from bregma; Medial-Lateral (ML), -1.2 mm from midline; and Dorsal-Ventral (DV), -3.4 mm from the skull surface [13]. Castration was also performed by the incision of the sac of the scrotum along with underlying tunica. The vas deferens was bilaterally tied off with a sterile chromic ligature, and both testes were removed; thereafter, incisions on the scrotum were sutured. The place of surgery was disinfected using betadine. Furthermore, the topical analgesic (2.5% lidocaine, 2.5% prilocaine) was applied to the incision site immediately after the surgery [14]. All procedures were approved by the local Animal Ethics Committee (EE/ 97, 24, 3061300/ scu.ac.ir).
Microinjection procedure
In this experimental study, male Wistar rats were divided into 10 groups. The experimental groups received DMSO as control groups and the different doses of ND (10, 30 and 60 µg/ 2.5 µL), anastrozole (2.5, 5 and 10 µg/ 2.5 µL), and anastrozole (2.5 µg/ 2.5 µL) + ND (60 µg/ 2.5 µL) all days before the training. The rats of ninth and tenth groups were castrated and treated with 2.5 µL of DMSO and ND (60 µg), respectively for 4 days.
Treatments initiated at 7 days (recovery time) after conducting the surgery. The intracerebral injection was administered through guide cannula (22-gauge) using injection needles (27-gauge) connected by polyethylene tubing to a Hamilton microsyringe. The injection needle was inserted 0.5 mm beyond the tip of the guide cannula. Then, vehicle (dimethyl sulfoxide, DMSO 10%) or different doses of ND and anastrozole were injected into the right lateral ventricle. All injections were performed at 30 min before testing, each day (during acquisition days). The other procedure of injection was administered for rats that were received two adjunct medications. In this procedure, anastrozole plus ND was injected with a 15-min interval. The injections (2.5 µL volume) were performed in 2.5 min and the injection needles were left in place for an additional minute. The drugs first dissolved in pure DMSO; then, the solution was diluted by the addition of saline solution to reach a final drug concentration.
Behavioral study
Spatial learning and memory were investigated using the Morris Water Maze (MWM) test. It consisted of a dark gray circular pool filled with water (24±1◦C) and a transparent platform submerged 1cm below the surface of the water. The pool was located in a room exclusively used for behavioral studies with a constant environment and visual cues on walls around the pool. Each trial was recorded and analyzed by a video camera connected to a tracking system (EthoVision XT) which was mounted above the pool. The test was designed in 4 trials for 4 consecutive days and probe trial was done on the fifth day.
The pool was divided into 4 parts, including north-west, northeast, south-west, and south-east. At the beginning of each trial, an animal was randomly placed facing the wall of the pool at one of 4 different starting points (north, east, south, and west). A maximum time of 60s was allowed to locate the hidden platform, which was situated in the north-west quadrant during all training trials. The rats were gently guided to the platform by the investigator, when not finding the platform within the maximum time. After locating, the platform rats were allowed to remain there for 30s. The platform was subsequently removed during the probe trial (at the fifth day) and the animals were allowed to search the platform location for 60s. The amount of time spent by the rats in target quadrants was determined [15].
Histology
At the end of the behavioral procedures, the injection site was histologically verified. The rats were deeply anesthetized and 2.5 µL of a solution of 4% methylene blue saline was infused into the implanted site; then, they were intracardially perfused with buffer phosphate saline, followed by 2% formalin. The rats’ brains were removed, the required sections were collected and the cannula tracks were examined for each rat. The subjects without adequate histological evidence were excluded from data processing (Figure 1) [16].
Experiments
Experiment 1: The effects of ND on spatial learning and memory in the MWM
The present experiment determined the effects of ND Intracerebroventricular (ICV) microinjection on MWM performance. The studied rats were divided into 4 groups according to the dose levels; 0 (n=11), 10(n=9), 30(n=10), and 60 µg (n=9) of ND dissolved in 2.5 µL of DMSO for 4 learning days.
Experiment 2: The effects of anastrozole on spatial learning and memory in the MWM
We also determined the effects of anastrozole as aromatase inhibitor on MWM performance. The studied rats were divided into 4 groups according to the dose levels; 0 (n=11), 2.5 (n=11), 5 (n=10), and 10 µg (n=11) of anastrozole dissolved in 2.5 µL DMSO for 4 learning days.
Experiment 3: The effects of the interaction of anastrozole and ND on spatial learning and memory in the MWM
We determined the role of P450 aromatase as a mediator of ND effects on spatial learning and memory. To evaluate this issue, anastrozole plus ND were ICV microinjected and the rats’ MWM performance was explored. The study rats were divided into 3 groups according to the dose levels of 0 µg+0 µg (n=11), 0 µg+60 µg (n=9) and 2.5 µg+60 µg (n=9) for anastrozole and ND, respectively for 4 learning days.
Experiment 4: The effects of the interaction of castration and ND on spatial learning and memory in the MWM
We evaluated the effects of castration on spatial learning and memory changes induced by ND. The study rats were divided into 4 groups. The first and second groups were sham-operated animals and respectively treated Intracerebro Ventricular Ventricle (ICV) with DMSO (2.5 µL, n=11) or 60 µg/ 2.5µL of ND (n=9) for 4 consecutive days. The rats of third and fourth groups were castrated and respectively treated with 2.5 µL of DMSO (n=9) or ND (60 µg, n=9) for 4 consecutive days.
Statistics
The MWM performances of animals during acquisition days were analyzed using Repeated Measures Analysis of Variance (R-ANOVA), followed by an LSD post hoc test to determine differences between the study groups. The traveled distance and escape latency of rats to reach the hidden platform could be affected by speed changes among groups; therefore, Analysis of Covariance (ANCOVA) was used with speed as a covariate [17]. For probe tests, the time spent in the target quadrant for each group was analyzed using one-way ANOVA. two-way R-ANOVA was conducted to analyze the effect of ND and castration (as independent variables) on escape latency and traveled distance to find the hidden platform. The effects of ND and castration on the times that rats spent in the target quadrant on the probe trial were analyzed using two-way ANOVA. The collected data are presented as a Mean±SD. Statistical significance was indicated by P<0.05.
3. Results
The effect of Nandrolone on MWM performance test
The effect of Nandrolone on spatial learning
Figure 2 showed the effect of ND (0, 10, 30, 60 µg/ 2.5 µL) on escape latency (A) and traveled distance (B). R-ANOVA revealed that ND, dose-dependently changed the escape latency (F3,144=4.613; P<0.05) and traveled distance (F3,144=3.738; P<0.05) during acquisition trials. Post hoc LSD analysis suggested that the escape latency and traveled distance decreased significantly in the group treated with 60 µg/ 2.5 µL of ND on the third and fourth days of training, compared to the control group (P<0.05). The insertion indicated the mean value of escape latency and traveled distance during the four days of acquisition trials. The mean value of escape latency and traveled distance in ND (60 µg) receivers was significantly lower than that of the controls (P<0.05).
The effect of Nandrolone on retention memory
The probe trials were conducted at 24 hours after the last acquisition day. One-way ANOVA demonstrated significant differences between the study groups (F3,36=4.367; P<0.05). Post hoc LSD analysis revealed that ND-treated animals (60 µg/ 2.5 µL) spent significantly more time in the target quadrant where the platform had previously been positioned, compared to the control group (P<0.05) (Figure 2C).
Experiment 3: The effects of the interaction of anastrozole and ND on spatial learning and memory in the MWM
We determined the role of P450 aromatase as a mediator of ND effects on spatial learning and memory. To evaluate this issue, anastrozole plus ND were ICV microinjected and the rats’ MWM performance was explored. The study rats were divided into 3 groups according to the dose levels of 0 µg+0 µg (n=11), 0 µg+60 µg (n=9) and 2.5 µg+60 µg (n=9) for anastrozole and ND, respectively for 4 learning days.
Experiment 4: The effects of the interaction of castration and ND on spatial learning and memory in the MWM
We evaluated the effects of castration on spatial learning and memory changes induced by ND. The study rats were divided into 4 groups. The first and second groups were sham-operated animals and respectively treated Intracerebro Ventricular Ventricle (ICV) with DMSO (2.5 µL, n=11) or 60 µg/ 2.5µL of ND (n=9) for 4 consecutive days. The rats of third and fourth groups were castrated and respectively treated with 2.5 µL of DMSO (n=9) or ND (60 µg, n=9) for 4 consecutive days.
Statistics
The MWM performances of animals during acquisition days were analyzed using Repeated Measures Analysis of Variance (R-ANOVA), followed by an LSD post hoc test to determine differences between the study groups. The traveled distance and escape latency of rats to reach the hidden platform could be affected by speed changes among groups; therefore, Analysis of Covariance (ANCOVA) was used with speed as a covariate [17]. For probe tests, the time spent in the target quadrant for each group was analyzed using one-way ANOVA. two-way R-ANOVA was conducted to analyze the effect of ND and castration (as independent variables) on escape latency and traveled distance to find the hidden platform. The effects of ND and castration on the times that rats spent in the target quadrant on the probe trial were analyzed using two-way ANOVA. The collected data are presented as a Mean±SD. Statistical significance was indicated by P<0.05.
3. Results
The effect of Nandrolone on MWM performance test
The effect of Nandrolone on spatial learning
Figure 2 showed the effect of ND (0, 10, 30, 60 µg/ 2.5 µL) on escape latency (A) and traveled distance (B). R-ANOVA revealed that ND, dose-dependently changed the escape latency (F3,144=4.613; P<0.05) and traveled distance (F3,144=3.738; P<0.05) during acquisition trials. Post hoc LSD analysis suggested that the escape latency and traveled distance decreased significantly in the group treated with 60 µg/ 2.5 µL of ND on the third and fourth days of training, compared to the control group (P<0.05). The insertion indicated the mean value of escape latency and traveled distance during the four days of acquisition trials. The mean value of escape latency and traveled distance in ND (60 µg) receivers was significantly lower than that of the controls (P<0.05).
The effect of Nandrolone on retention memory
The probe trials were conducted at 24 hours after the last acquisition day. One-way ANOVA demonstrated significant differences between the study groups (F3,36=4.367; P<0.05). Post hoc LSD analysis revealed that ND-treated animals (60 µg/ 2.5 µL) spent significantly more time in the target quadrant where the platform had previously been positioned, compared to the control group (P<0.05) (Figure 2C).
The Effects of anastrozole on MWM performance test
The Effects of anastrozole on spatial learning
Repeated measures ANOVA suggested significant differences in escape latency (F3,164=4.041; P<0.05, Figure 3A) and traveled distance (F3,164=5.189; P<0.05, Figure 3B) among the study groups. Post hoc LSD analysis demonstrated that the escape latency and traveled distance significantly decreased in the anastrozole-treated group, compared to the controls (P<0.05). The insertion indicated the mean of escape latency and traveled distance during the four-day acquisition trials. The mean value of escape latency and traveled distance of anastrozole (2.5, 5 and 10 µg/ 2.5 µL) group was significantly lower than that of the control group (P<0.05).
The Effects of anastrozole on spatial learning
Repeated measures ANOVA suggested significant differences in escape latency (F3,164=4.041; P<0.05, Figure 3A) and traveled distance (F3,164=5.189; P<0.05, Figure 3B) among the study groups. Post hoc LSD analysis demonstrated that the escape latency and traveled distance significantly decreased in the anastrozole-treated group, compared to the controls (P<0.05). The insertion indicated the mean of escape latency and traveled distance during the four-day acquisition trials. The mean value of escape latency and traveled distance of anastrozole (2.5, 5 and 10 µg/ 2.5 µL) group was significantly lower than that of the control group (P<0.05).
The Effects of anastrozole on retention memory
One-way ANOVA demonstrated no significant difference in the time spent in the target quadrant between control and anastrozole-treated groups (Figure 3C).
The effects of the interaction of anastrozole and Nandrolone on MWM performance test
The effects of the interaction of anastrozole and Nandrolone on spatial learning
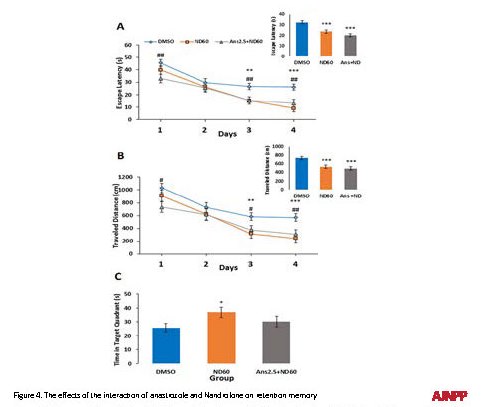
R-ANOVA was conducted to examine the effect of anastrozole plus ND on escape latency and traveled distance to find the hidden platform. The obtained data suggested significant differences in escape latency (F2,105=11.813; P<0.05, Figure 4A) and traveled distance (F2,105= 9.159; P<0.05, Figure 4B) among the groups. Post hoc LSD analysis indicated that the mean value of escape latency and traveled distance of anastrozole plus ND was lower than that of the control group on the first, third and fourth days of training (P<0.05). The insertion revealed a mean of escape latency and traveled distance during the four-day acquisition trials. The mean value of escape latency and traveled distance of anastrozole (2.5 µg) plus ND (60 µg) treated groups was significantly lower than that of the controls (P<0.05). Therefore, the pretreatment of anastrozole (2.5 µg) has no effect on ND-induced spatial learning improvement.
One-way ANOVA demonstrated no significant difference in the time spent in the target quadrant between control and anastrozole-treated groups (Figure 3C).
The effects of the interaction of anastrozole and Nandrolone on MWM performance test
The effects of the interaction of anastrozole and Nandrolone on spatial learning
R-ANOVA was conducted to examine the effect of anastrozole plus ND on escape latency and traveled distance to find the hidden platform. The obtained data suggested significant differences in escape latency (F2,105=11.813; P<0.05, Figure 4A) and traveled distance (F2,105= 9.159; P<0.05, Figure 4B) among the groups. Post hoc LSD analysis indicated that the mean value of escape latency and traveled distance of anastrozole plus ND was lower than that of the control group on the first, third and fourth days of training (P<0.05). The insertion revealed a mean of escape latency and traveled distance during the four-day acquisition trials. The mean value of escape latency and traveled distance of anastrozole (2.5 µg) plus ND (60 µg) treated groups was significantly lower than that of the controls (P<0.05). Therefore, the pretreatment of anastrozole (2.5 µg) has no effect on ND-induced spatial learning improvement.
The effects of the interaction of anastrozole and Nandrolone on retention memory
One-way ANOVA demonstrated that the time spent in the target quadrants did not significantly change between the control and anastrozole (2.5 µg) puls ND (60 µg) groups (Figure 4C).
The effects of the interaction of castration and Nandrolone on MWM performance test
The effects of the interaction of castration and Nandrolone on spatial learning
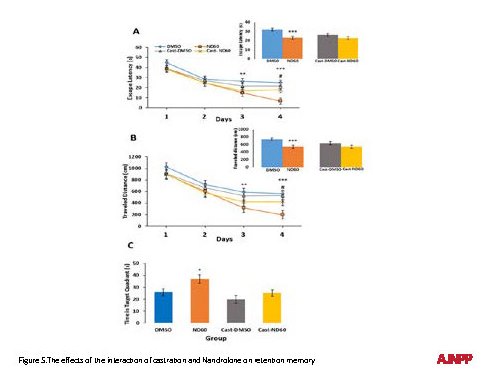
The two-way R-ANOVA was conducted to examine the effect of ND (60 µg) and castration on escape latency and traveled distance to find the hidden platform. There was significant differences in escape latency (F1,140=4.722; P<0.05, Figure 5A) and traveled distance (F1,140=3.330; P<0.05, Figure 5B) among the study groups. The pairwise comparison suggested that escape latency and traveled distance in the group of castration which received ND (Cast-ND60) increased significantly on the fourth day of training, compared to the ND (60 µg) treated group (P<0.05). The insertion indicated the mean value of escape latency and traveled distance during the four-day acquisition trials. There was no significant effect on the control and Cast-ND60 groups, which suggested that the castration could be buffered by ND60-induced improvement effect.
The effects of the interaction of castration and Nandrolone on retention memory
Two-way ANOVA demonstrated that ND60-treated animals (60 µg/ 2.5µL) spent significantly more time in the target quadrant where the platform had previously been positioned (P<0.05); the changes of other groups were not significant (Figure 5C).
Effects of Nandrolone, anastrozole, and castration on swimming speed
One-way ANOVA demonstrated that the time spent in the target quadrants did not significantly change between the control and anastrozole (2.5 µg) puls ND (60 µg) groups (Figure 4C).
The effects of the interaction of castration and Nandrolone on MWM performance test
The effects of the interaction of castration and Nandrolone on spatial learning
The two-way R-ANOVA was conducted to examine the effect of ND (60 µg) and castration on escape latency and traveled distance to find the hidden platform. There was significant differences in escape latency (F1,140=4.722; P<0.05, Figure 5A) and traveled distance (F1,140=3.330; P<0.05, Figure 5B) among the study groups. The pairwise comparison suggested that escape latency and traveled distance in the group of castration which received ND (Cast-ND60) increased significantly on the fourth day of training, compared to the ND (60 µg) treated group (P<0.05). The insertion indicated the mean value of escape latency and traveled distance during the four-day acquisition trials. There was no significant effect on the control and Cast-ND60 groups, which suggested that the castration could be buffered by ND60-induced improvement effect.
The effects of the interaction of castration and Nandrolone on retention memory
Two-way ANOVA demonstrated that ND60-treated animals (60 µg/ 2.5µL) spent significantly more time in the target quadrant where the platform had previously been positioned (P<0.05); the changes of other groups were not significant (Figure 5C).
Effects of Nandrolone, anastrozole, and castration on swimming speed
Figure 6. showed the mean value of swimming speed during the four-day acquisition trials. The analysis suggested that swimming speed was significantly changed in the groups which received ND (10 µg) (P<0.05) and anastrozole (5 µg) (P<0.001), compared to the control group (Figure 6A). Additionally, swimming speed changes in the groups of castration which received DMSO (Cast-DMSO) (P<0.01) and ND (60 µg) (Cast-ND60) (P<0.001) were significant, compared to the control group (Figure 6B). The traveled distance and escape latency of rats to reach the hidden platform could be affected by speed changes among groups; therefore, ANCOVA was used with speed as a covariate [17]. To exclude the effect of speed changes, we used speed as a Covariate in Statistical Analysis (ANCOVA) [17].
4. Discussion
Most studies have mainly examined the misuse of AASs in adults, while adolescents have received little attention. Furthermore, adolescents are more susceptible to the deleterious effects of AASs, than adults [2]. Therefore, the present study was the first to address cognitive effects on spatial learning and memory in adolescent male rats after exposure to ND (ICV injection).
4. Discussion
Most studies have mainly examined the misuse of AASs in adults, while adolescents have received little attention. Furthermore, adolescents are more susceptible to the deleterious effects of AASs, than adults [2]. Therefore, the present study was the first to address cognitive effects on spatial learning and memory in adolescent male rats after exposure to ND (ICV injection).
We demonstrated that the subchronic daily ICV microinjection of ND improved the spatial learning and memory of adolescent male rats. However, previous studies have reported that the chronic systemic administration of ND impairs spatial learning and memory of adult male rats [1, 18]. This discrepancy may arise from the differences between the treatment protocols of the studies. In addition, the chronic systemic usage of ND may expose CNS to a high level of ND for a longer time than a small quantity injection of ND into cerebral ventricle which might expose CNS to a high acute dose of ND. The later might rapidly disappear from the brain by dissolving in total body fluid [19]. This finding is in line with the studies reporting that adolescents are more sensitive to the effects of androgens [20]. Moreover, the changing testosterone levels during puberty are significantly associated with behavior in adolescent males. It is also well established that adolescence is a sensitive developmental period that underscores the potential for effects of AASs, compared to adulthood [2].
Furthermore, Rossbach et al. reported that a single high dose of AAS nandrolone increases the phosphorylation of both NMDAR and ERK 1/2 in hippocampal synaptoneurosomes. In addition, they reported a single injection of nandrolone can affect important synaptic components involved in hippocampal plasticity [21]. On the other hand, studies have described that some steroids are the negative modulators of the GABAA receptor and positive modulators of the N-methyl-D aspartate (NMDA) [2]. A large body of experiments has studied the mechanism of endogenous androgens effect on animal cognitive performance [6, 22].
However, a previous study argued that the effect of ND on memory is induced through the stimulation of AR. The underlying mechanism of ND effects on learning and memory are complex and have remained unclear. Additionally, the aromatization of ND to estrogen could be one of the mechanisms behind the effects of ND on spatial learning and memory. Therefore, the effect of ND on spatial learning and memory through the induction of p450 aromatase enzyme was explored applying an aromatase inhibitor, anastrozole. The obtained data revealed that anastrozole improves spatial learning which is in accordance with a previous study [6]. However, anastrozole could not change the ND effect on spatial learning and memory and our hypothesis was rejected, indicating that ND has no interaction with aromatase pathways regarding spatial learning and memory.
Furthermore, prior investigations have reported that ND can be aromatized to estrogen, but to a lesser extent [3]. It is possible to inhibit the aromatase enzyme as a result; low levels of estrogen prohibit negative feedback from intermediate enzymes. Thus, by increasing the production of estrogen precursors, such as pregnenolone, Dehydroepiandrosterone (DHEA) and DHEA-sulphate (DHEA-S), learning and memory may improve. Extrinsic androgens could also affect the hypothalamus-hypophysis-gonadal function and inhibit or induce endogenic testosterone secretion depending on the treatment duration [11, 23, 24]. An optimal level of endogenous testosterone is essential for normal cognitive performances [7, 12]. We previously demonstrated that the intraventricular injection of ND increased testosterone concentration [25].
Therefore, ND may affect spatial learning and memory through making changes in intrinsic gonadal hormone level. To evaluate the role of gonadal function on spatial learning and memory, the studied rats were castrated and their spatial learning and memory were tested one week later. The relevant data suggested that castration had no effect on spatial learning; however, it abolished the improvement effect of ND, indicating that at least some effect of ND on learning, induced through changes in gonadal function. Several reports consistently indicated that castration does not significantly disturb acquisition or probe trial performance in a classic reference memory task [14, 26, 27], which is in agreement with the present study. We used the intracerebroventricular microinjection of ND to avoid the peripheral action of ND; therefore, it should be considered that this protocol is different from the human abuse of ND and the results cannot be directly related to usual ND abuse effects.
5. Conclusion
This study has revealed that although ND administration could affect spatial learning and memory, its effect could be prevented by castration. Therefore, ND affects spatial learning via changes in the circulating levels of testosterone. These findings may contribute to the understanding of the underlying biological pathways that lead to the cognitive changes associated with ND in humans. However, the mechanisms need future investigations.
Ethical Considerations
Compliance with ethical guidelines
All procedures were approved by the local Animal Ethics Committee (EE/ 97, 24, 3061300/ scu.ac.ir).
Funding
This research was supported by the Shahid Chamran University of Ahvaz.
Authors' contributions
Investigation and preparing orginal draft: Zahra Salimi; Supervasion, resource and analysis: Farshad Moradpour; Supervision and resource: Lotfollah Khajehpour; Editing: Ahmad Ali Moazedi and Ali ppourmotabbed.
Conflict of interest
The authors declared no conflicts of interests.
Acknowledgments
The authors would like to acknowledge the Shahid Chamran University of Ahvaz and Kermanshah University of Medical Sciences to support this work.
References
Magnusson K, Hånell A, Bazov I, Clausen F, Zhou Q, Nyberg F. Nandrolone decanoate administration elevates hippocampal prodynorphin mRNA expression and impairs morris water maze performance in male rats. Neuroscience Letters. 2009; 467(3):189-93. [DOI:10.1016/j.neulet.2009.09.041] [PMID]
Basaria SW, Dobs AS. Anabolic-androgenic steroid therapy in the treatment of chronic diseases. The Journal of Clinical Endocrinology and Metabolism. 2001; 86(11):5108-17. [DOI:10.1210/jcem.86.11.7983] [PMID]
Mottram DR, George AJ. Anabolic steroids. Best Practice & Research Clinical Endocrinology & Metabolism. 2000; 14(1):55-69. [DOI:10.1053/beem.2000.0053]
Kouvelas D, Pourzitaki C, Papazisis G, Dagklis T, Dimou K, Kraus MM. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. International Journal of Neuropsychopharmacology. 2008; 11(7):925-34. [DOI:10.1017/S1461145708008754]
Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the morris water maze. Brain Research. 2001; 897(1-2):44-51. [DOI:10.1016/S0006-8993(00)03261-3]
Moradpour F, Naghdi N, Fathollahi Y. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behavioural Brain Research. 2006; 175(2):223-32. [DOI:10.1016/j.bbr.2006.08.037] [PMID]
Babanejad S, Naghdi N, Rohani SA. Microinjection of dihydrotestosterone as a 5α-reduced metabolite of testosterone into ca1 region of hippocampus could improve spatial learning in the adult male rats. Iranian journal of Pharmaceutical Research. 2012; 11(2):661-9. [PMID] [PMCID]
Nayebi AM, Pourrabi S, Hossini S. Testosterone ameliorates streptozotocin-induced memory impairment in male rats. Acta Pharmacologica Sinica. 2014; 35(6):752-7. [DOI:10.1038/aps.2014.6] [PMID] [PMCID]
Ryan KJ. Biological aromatization of steroids. Journal of Biological Chemistry. 1959; 234(2):268-72. [PMID]
Roselli CE. The effect of anabolic- androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Research. 1998; 792(2):271-6. [DOI:10.1016/S0006-8993(98)00148-6]
Takahashi M, Tatsugi Y, Kohno T. Endocrinological and pathological effects of anabolic-androgenic steroid in male rats. Endocrine Journal. 2004; 51(4):425-34. [DOI:10.1507/endocrj.51.425] [PMID]
Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral Neuroscience. 1994; 108(2):325-32. [DOI:10.1037//0735-7044.108.2.325] [PMID]
Paxinos G, Franklin KB. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. Cambridge: Academic Press; 2019.
Spritzer MD, Gill M, Weinberg A, Galea LA. Castration differentially affects spatial working and reference memory in male rats. Archives of Sexual Behavior. 2008; 37(1):19-29. [DOI:10.1007/s10508-007-9264-2] [PMID]
Pourmotabbed A, Nedaei SE, Cheraghi M, Moradian S, Touhidi A, Aeinfar M, et al. Effect of prenatal pentylenetetrazol-induced kindling on learning and memory of male offspring. Neuroscience. 2011; 172:205-11. [DOI:10.1016/j.neuroscience.2010.11.001] [PMID]
Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Research. 1996; 730(1-2):32-9. [DOI:10.1016/0006-8993(96)00427-1]
Moradpour F, Naghdi N, Fathollahi Y, Javan M, Choopani S, Gharaylou Z. Pre-pubertal castration improves spatial learning during mid-adolescence in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013; 46:105-12. [DOI:10.1016/j.pnpbp.2013.07.005] [PMID]
Karamian A, Pakdel FG, Ilkhanipoor M, Farokhi F, Ahmadi A. An intra-hippocampal injection of nandrolone induces learning and memory impairments in rat. Drug Research. 2015; 65(01):1-4. [DOI:10.1055/s-0033-1364000] [PMID]
Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids and Barriers of the CNS. 2011; 8:7. [DOI:10.1186/2045-8118-8-7] [PMID] [PMCID]
Lumia AR, McGinnis MY. Impact of anabolic androgenic steroids on adolescent males. Physiology & Behavior. 2010; 100(3):199-204. [DOI:10.1016/j.physbeh.2010.01.007] [PMID]
Rossbach UL, Steensland P, Nyberg F, Le Grevès P. Nandrolone-induced hippocampal phosphorylation of NMDA receptor subunits and ERKs. Biochemical and Biophysical Research Communications. 2007; 357(4):1028-33. [DOI:10.1016/j.bbrc.2007.04.037] [PMID]
Naghdi N, Asadollahi A. Genomic and nongenomic effects of intrahippocampal microinjection of testosterone on long-term memory in male adult rats. Behavioural Brain Research. 2004; 153(1):1-6. [DOI:10.1016/j.bbr.2003.10.027] [PMID]
Jannatifar R, Shokri S, Farrokhi A, Nejatbakhsh R. Effect of supraphysiological dose of Nandrolone Decanoate on the testis and testosterone concentration in mature and immature male rats: A time course study. International Journal of Reproductive BioMedicine. 2015; 13(12):779-86. [DOI:10.29252/ijrm.13.12.779] [PMID] [PMCID]
Purkayastha S, Mahanta R. Effect of nandrolone decanoate on serum FSH, LH and testosterone concentration in male Albino mice. World Journal of Life Sciences and Medical Research. 2012; 2(3):123-7.
Zarei F, Moradpour F, Moazedi AA, Pourmotabbed A, Veisi M. Nandrolone administration abolishes hippocampal fEPSP-PS potentiation and passive avoidance learning of adolescent male rats. Canadian Journal of Physiology and Pharmacology. 2018; 97(2):130-9. [DOI:10.1139/cjpp-2018-0293]
Mohaddes G, Naghdi N, Khamnei S, Khatami S, Haeri A. Effect of spatial learning on hippocampal testosterone in intact and castrated male rats. Iranian Biomedical Journal. 2009; 13(1):49-58. [PMID]
Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Hormones and Behavior. 2006; 50(1):18-26. [DOI:10.1016/j.yhbeh.2005.09.008] [PMID]
Furthermore, Rossbach et al. reported that a single high dose of AAS nandrolone increases the phosphorylation of both NMDAR and ERK 1/2 in hippocampal synaptoneurosomes. In addition, they reported a single injection of nandrolone can affect important synaptic components involved in hippocampal plasticity [21]. On the other hand, studies have described that some steroids are the negative modulators of the GABAA receptor and positive modulators of the N-methyl-D aspartate (NMDA) [2]. A large body of experiments has studied the mechanism of endogenous androgens effect on animal cognitive performance [6, 22].
However, a previous study argued that the effect of ND on memory is induced through the stimulation of AR. The underlying mechanism of ND effects on learning and memory are complex and have remained unclear. Additionally, the aromatization of ND to estrogen could be one of the mechanisms behind the effects of ND on spatial learning and memory. Therefore, the effect of ND on spatial learning and memory through the induction of p450 aromatase enzyme was explored applying an aromatase inhibitor, anastrozole. The obtained data revealed that anastrozole improves spatial learning which is in accordance with a previous study [6]. However, anastrozole could not change the ND effect on spatial learning and memory and our hypothesis was rejected, indicating that ND has no interaction with aromatase pathways regarding spatial learning and memory.
Furthermore, prior investigations have reported that ND can be aromatized to estrogen, but to a lesser extent [3]. It is possible to inhibit the aromatase enzyme as a result; low levels of estrogen prohibit negative feedback from intermediate enzymes. Thus, by increasing the production of estrogen precursors, such as pregnenolone, Dehydroepiandrosterone (DHEA) and DHEA-sulphate (DHEA-S), learning and memory may improve. Extrinsic androgens could also affect the hypothalamus-hypophysis-gonadal function and inhibit or induce endogenic testosterone secretion depending on the treatment duration [11, 23, 24]. An optimal level of endogenous testosterone is essential for normal cognitive performances [7, 12]. We previously demonstrated that the intraventricular injection of ND increased testosterone concentration [25].
Therefore, ND may affect spatial learning and memory through making changes in intrinsic gonadal hormone level. To evaluate the role of gonadal function on spatial learning and memory, the studied rats were castrated and their spatial learning and memory were tested one week later. The relevant data suggested that castration had no effect on spatial learning; however, it abolished the improvement effect of ND, indicating that at least some effect of ND on learning, induced through changes in gonadal function. Several reports consistently indicated that castration does not significantly disturb acquisition or probe trial performance in a classic reference memory task [14, 26, 27], which is in agreement with the present study. We used the intracerebroventricular microinjection of ND to avoid the peripheral action of ND; therefore, it should be considered that this protocol is different from the human abuse of ND and the results cannot be directly related to usual ND abuse effects.
5. Conclusion
This study has revealed that although ND administration could affect spatial learning and memory, its effect could be prevented by castration. Therefore, ND affects spatial learning via changes in the circulating levels of testosterone. These findings may contribute to the understanding of the underlying biological pathways that lead to the cognitive changes associated with ND in humans. However, the mechanisms need future investigations.
Ethical Considerations
Compliance with ethical guidelines
All procedures were approved by the local Animal Ethics Committee (EE/ 97, 24, 3061300/ scu.ac.ir).
Funding
This research was supported by the Shahid Chamran University of Ahvaz.
Authors' contributions
Investigation and preparing orginal draft: Zahra Salimi; Supervasion, resource and analysis: Farshad Moradpour; Supervision and resource: Lotfollah Khajehpour; Editing: Ahmad Ali Moazedi and Ali ppourmotabbed.
Conflict of interest
The authors declared no conflicts of interests.
Acknowledgments
The authors would like to acknowledge the Shahid Chamran University of Ahvaz and Kermanshah University of Medical Sciences to support this work.
References
Magnusson K, Hånell A, Bazov I, Clausen F, Zhou Q, Nyberg F. Nandrolone decanoate administration elevates hippocampal prodynorphin mRNA expression and impairs morris water maze performance in male rats. Neuroscience Letters. 2009; 467(3):189-93. [DOI:10.1016/j.neulet.2009.09.041] [PMID]
Basaria SW, Dobs AS. Anabolic-androgenic steroid therapy in the treatment of chronic diseases. The Journal of Clinical Endocrinology and Metabolism. 2001; 86(11):5108-17. [DOI:10.1210/jcem.86.11.7983] [PMID]
Mottram DR, George AJ. Anabolic steroids. Best Practice & Research Clinical Endocrinology & Metabolism. 2000; 14(1):55-69. [DOI:10.1053/beem.2000.0053]
Kouvelas D, Pourzitaki C, Papazisis G, Dagklis T, Dimou K, Kraus MM. Nandrolone abuse decreases anxiety and impairs memory in rats via central androgenic receptors. International Journal of Neuropsychopharmacology. 2008; 11(7):925-34. [DOI:10.1017/S1461145708008754]
Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the morris water maze. Brain Research. 2001; 897(1-2):44-51. [DOI:10.1016/S0006-8993(00)03261-3]
Moradpour F, Naghdi N, Fathollahi Y. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behavioural Brain Research. 2006; 175(2):223-32. [DOI:10.1016/j.bbr.2006.08.037] [PMID]
Babanejad S, Naghdi N, Rohani SA. Microinjection of dihydrotestosterone as a 5α-reduced metabolite of testosterone into ca1 region of hippocampus could improve spatial learning in the adult male rats. Iranian journal of Pharmaceutical Research. 2012; 11(2):661-9. [PMID] [PMCID]
Nayebi AM, Pourrabi S, Hossini S. Testosterone ameliorates streptozotocin-induced memory impairment in male rats. Acta Pharmacologica Sinica. 2014; 35(6):752-7. [DOI:10.1038/aps.2014.6] [PMID] [PMCID]
Ryan KJ. Biological aromatization of steroids. Journal of Biological Chemistry. 1959; 234(2):268-72. [PMID]
Roselli CE. The effect of anabolic- androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Research. 1998; 792(2):271-6. [DOI:10.1016/S0006-8993(98)00148-6]
Takahashi M, Tatsugi Y, Kohno T. Endocrinological and pathological effects of anabolic-androgenic steroid in male rats. Endocrine Journal. 2004; 51(4):425-34. [DOI:10.1507/endocrj.51.425] [PMID]
Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral Neuroscience. 1994; 108(2):325-32. [DOI:10.1037//0735-7044.108.2.325] [PMID]
Paxinos G, Franklin KB. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. Cambridge: Academic Press; 2019.
Spritzer MD, Gill M, Weinberg A, Galea LA. Castration differentially affects spatial working and reference memory in male rats. Archives of Sexual Behavior. 2008; 37(1):19-29. [DOI:10.1007/s10508-007-9264-2] [PMID]
Pourmotabbed A, Nedaei SE, Cheraghi M, Moradian S, Touhidi A, Aeinfar M, et al. Effect of prenatal pentylenetetrazol-induced kindling on learning and memory of male offspring. Neuroscience. 2011; 172:205-11. [DOI:10.1016/j.neuroscience.2010.11.001] [PMID]
Lorenzini CA, Baldi E, Bucherelli C, Sacchetti B, Tassoni G. Role of dorsal hippocampus in acquisition, consolidation and retrieval of rat’s passive avoidance response: A tetrodotoxin functional inactivation study. Brain Research. 1996; 730(1-2):32-9. [DOI:10.1016/0006-8993(96)00427-1]
Moradpour F, Naghdi N, Fathollahi Y, Javan M, Choopani S, Gharaylou Z. Pre-pubertal castration improves spatial learning during mid-adolescence in rats. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013; 46:105-12. [DOI:10.1016/j.pnpbp.2013.07.005] [PMID]
Karamian A, Pakdel FG, Ilkhanipoor M, Farokhi F, Ahmadi A. An intra-hippocampal injection of nandrolone induces learning and memory impairments in rat. Drug Research. 2015; 65(01):1-4. [DOI:10.1055/s-0033-1364000] [PMID]
Pardridge WM. Drug transport in brain via the cerebrospinal fluid. Fluids and Barriers of the CNS. 2011; 8:7. [DOI:10.1186/2045-8118-8-7] [PMID] [PMCID]
Lumia AR, McGinnis MY. Impact of anabolic androgenic steroids on adolescent males. Physiology & Behavior. 2010; 100(3):199-204. [DOI:10.1016/j.physbeh.2010.01.007] [PMID]
Rossbach UL, Steensland P, Nyberg F, Le Grevès P. Nandrolone-induced hippocampal phosphorylation of NMDA receptor subunits and ERKs. Biochemical and Biophysical Research Communications. 2007; 357(4):1028-33. [DOI:10.1016/j.bbrc.2007.04.037] [PMID]
Naghdi N, Asadollahi A. Genomic and nongenomic effects of intrahippocampal microinjection of testosterone on long-term memory in male adult rats. Behavioural Brain Research. 2004; 153(1):1-6. [DOI:10.1016/j.bbr.2003.10.027] [PMID]
Jannatifar R, Shokri S, Farrokhi A, Nejatbakhsh R. Effect of supraphysiological dose of Nandrolone Decanoate on the testis and testosterone concentration in mature and immature male rats: A time course study. International Journal of Reproductive BioMedicine. 2015; 13(12):779-86. [DOI:10.29252/ijrm.13.12.779] [PMID] [PMCID]
Purkayastha S, Mahanta R. Effect of nandrolone decanoate on serum FSH, LH and testosterone concentration in male Albino mice. World Journal of Life Sciences and Medical Research. 2012; 2(3):123-7.
Zarei F, Moradpour F, Moazedi AA, Pourmotabbed A, Veisi M. Nandrolone administration abolishes hippocampal fEPSP-PS potentiation and passive avoidance learning of adolescent male rats. Canadian Journal of Physiology and Pharmacology. 2018; 97(2):130-9. [DOI:10.1139/cjpp-2018-0293]
Mohaddes G, Naghdi N, Khamnei S, Khatami S, Haeri A. Effect of spatial learning on hippocampal testosterone in intact and castrated male rats. Iranian Biomedical Journal. 2009; 13(1):49-58. [PMID]
Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Hormones and Behavior. 2006; 50(1):18-26. [DOI:10.1016/j.yhbeh.2005.09.008] [PMID]
Article Type: Research Article |
Subject:
Learning and Memory, Dementia, Alzheimer
Received: 2017/03/12 | Accepted: 2017/08/29 | Published: 2017/11/1
Received: 2017/03/12 | Accepted: 2017/08/29 | Published: 2017/11/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |