Volume 9, Issue 4 (November 2022)
Avicenna J Neuro Psycho Physiology 2022, 9(4): 150-162 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Joodaki M, Radahmadi M. Depression and Different Brain Areas: Neural Activity and Potential Mechanisms. Avicenna J Neuro Psycho Physiology 2022; 9 (4) :150-162
URL: http://ajnpp.umsha.ac.ir/article-1-434-en.html
URL: http://ajnpp.umsha.ac.ir/article-1-434-en.html
1- Department of Physiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
2- Department of Physiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran , m_radahmadi@med.mui.ac.ir
2- Department of Physiology, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran , m_radahmadi@med.mui.ac.ir
Full-Text [PDF 3393 kb]
(258 Downloads)
| Abstract (HTML) (851 Views)

Figure1. Various psychological, behavioral, and physiological symptoms related to depression

Figure2. A schematic diagram of depression and the involved systems [114]
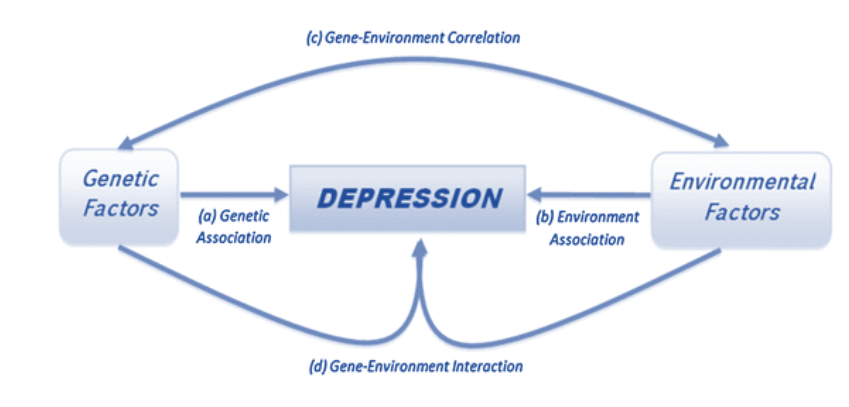
Figure3. Etiological paradigm of depression [115]
Unfortunately, genetic and epigenetic modifications require high costs. However, improvement in environmental factors seems to have decreased depression in subjects. Therefore, eliminating those environmental factors that caused and aggravated depression (i.e., stress, nutrition, etc.) could contribute to alleviation of depression and faster improvement in its treatment process.
Stress activates the HPA axis and sympathetic nervous system leading to the release of glucocorticoids and catecholamines into the blood [25]. Long exposure to glucocorticoids has neurotoxic effects on the brain [26]. Therefore, it could cause many neurological and psychological diseases similar to depression. Many studies have confirmed the relationship between depression and stress-related structural changes in the brain that alter mood and cause physical dysfunctions [4, 27, 28]. Therefore, chronic stress, which changes the body’s homeostasis, could eventually lead to depression or worsened symptoms of depression.
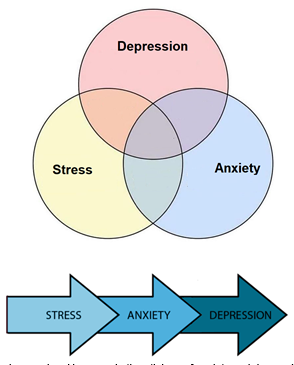
Figure 4. Stress, anxiety, and depression overlap. However, in the etiology of anxiety and depression, psychological stress is a major risk factor [54]

Figure 5. A conceptual scheme for the major brain network circuit involved in depression-related behaviors and neurotransmitter secretion from different brain nuclei [116]
Patients with MDD had a smaller volume of the medial orbitofrontal cortex (mOFC or gyrus rectus) [35]. The activity in the dorsal system [hippocampus, dorsolateral PFC (DL-PFC), and dorsal anterior cingulate cortex (dACC)] is reduced in depression. In similar conditions, increased activity has been observed in the ventral system (amygdala, insula, ventral striatum, subgenual cingulate cortex, ventral parts of the ACC and PFC) [34, 36].
In people with depression, morphological alterations, including neural atrophy, reduced number of glial cells, dendritic spines, brain metabolism, brain volume, and dendritic arborization have been reported in several cortical and limbic areas like PFC and hippocampus [8, 25, 37]. Similarly, some changes were observed in the gray matter volume, neural organization, electro-physiological activity, and neurotransmitter receptors in the mentioned brain regions [34]. As shown in Figure 7, despite the hyperactivation of some brain areas in depression, other regions may become hypoactive [38]. As such, several brain regions changed in patients with depression, including the limbic system, hippocampus,
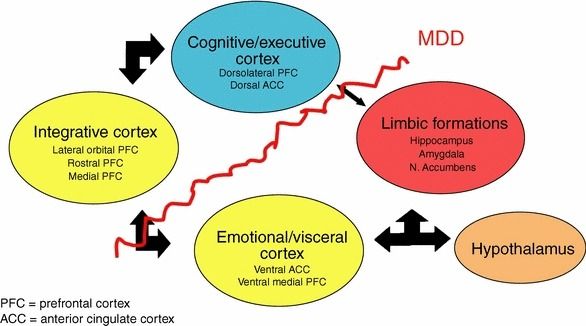
Figure 6. Limbic regions have reciprocal connections with the “para-limbic” cortical areas, subgenual anterior cingulate, and vmPFC [117]

Figure 7. Some brain areas are hypoactive, whereas other regions are hyperactive in depression
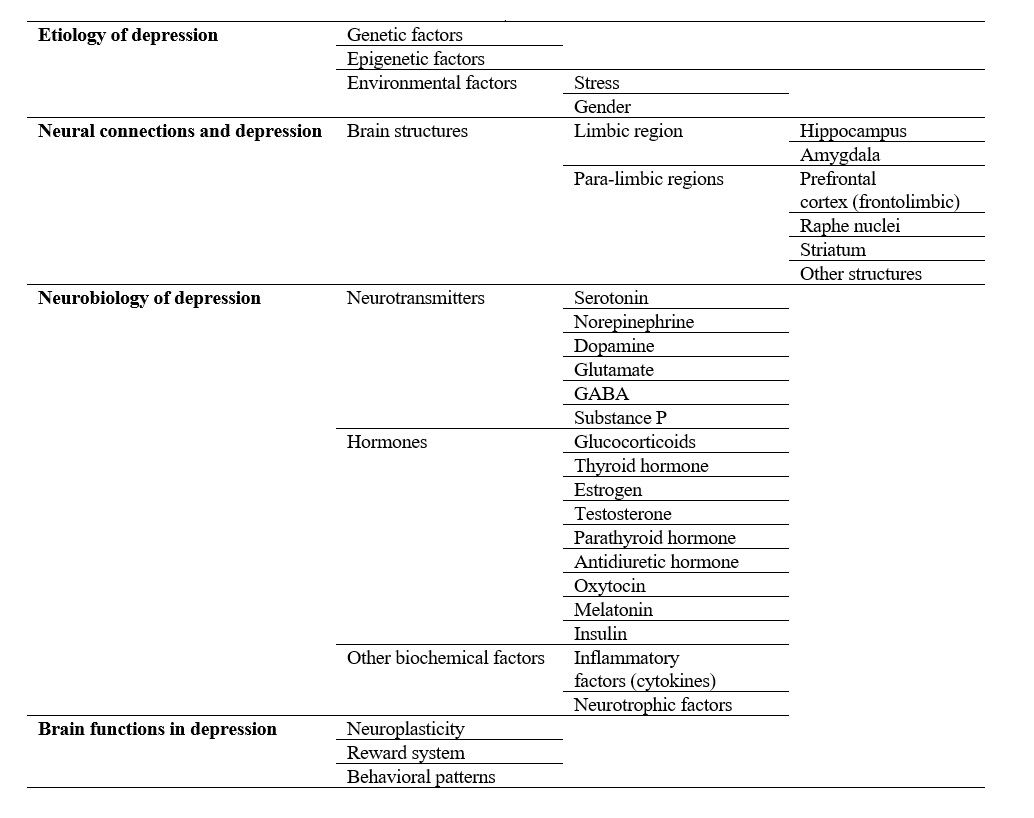
Figure 8. A summary diagram of how depression affects different brain aspects
Full-Text: (866 Views)
Background
Major depressive disorder (MDD), also known as depression, is a common psychological disorder [1] and is indicated as one of the most prevailing problems in today’s society [2]. Its global prevalence has increased to 320 million suffering from MDD [3]. As shown in Figure1, its most common symptoms include cognitive dysfunctions (e.g., impaired learning and memory), mood swings [4, 5], continuous low mood, feelings of worthlessness, anhedonia, social withdrawal, sleep disturbance, and suicidal thoughts [6, 7].

Figure1. Various psychological, behavioral, and physiological symptoms related to depression
Various factors, such as cellular, neurochemical, and neuroendocrine impairments, may cause depression [8]. Therefore, different methods are developed for its treatment, such as self-help, exercise, talking therapy, interpersonal psychotherapy, use of medi-cations and medicinal plants, and polytherapy [9-12]. However, proper and effective treatment for depression requires a comprehensive knowledge of its causes. This is why in-depth knowledge about the main factors behind this disorder leads to better treatment choices. The overview of the role different brain areas and some of their influencing factors play could be beneficial for the treatment of depression (fig. 2); this study has thus attempted to provide an integrated summary of various brain areas and factors involved in depression.
Etiology of depression
Depression is a complex disorder with a combination of genetic, epigenetic, and environmental factors that lead to developing depression [8] (fig. 3).Genetic factors and depression
Numerous family, twin, and adoption-related studies suggest a high potential for genetic predisposition to MDD [13]. Therefore, compared to early life experiences, genetic vulnerability seems to have a more significant role in developing depression [14]. In addition, reduced expression of the serotonin transporter gene increases the risk factor [15]. Moreover, apolipoprotein E4, DE4A, FDX1L, and MYO15B genes are linked to the potential risk of genetic depression [16, 17].Epigenetic factors and depression
Several studies have indicated the role of prenatal and postnatal epigenetic factors in the neurodevelopment of different brain regions [hypothalamus, hippo-campus, prefrontal cortex (PFC), and amygdala], neuroendocrine systems, behavior (depression or anxiety), and cognition (learning and memory) [8]. Early prenatal stress impairs corticotropin-releasing hormone (CRH), which in turn increases hypothalamic-pituitary-adrenal (HPA) axis activity and changes the serotonin system [18]. Thus, environ-mental factors during childhood could modify neural circuits, functions, and gene expression [8]. The neuroanatomical, neurochemical, and behavioral changes in depressed individuals could originate from the DNA methylation alterations in early life experiences [8].
Figure2. A schematic diagram of depression and the involved systems [114]

Figure3. Etiological paradigm of depression [115]
Environmental factors and depression
Different risk factors, including postnatal factors, cause depression among sociable humans. For instance, chronic stress diseases as the main factor, alcohol abuse, social isolation and deprivation, prior depressive episodes, low birth weight, malnutrition, and vitamin deficiency, particularly insufficiency of vitamin D and B12, could result in depression [5, 8, 19-21].Unfortunately, genetic and epigenetic modifications require high costs. However, improvement in environmental factors seems to have decreased depression in subjects. Therefore, eliminating those environmental factors that caused and aggravated depression (i.e., stress, nutrition, etc.) could contribute to alleviation of depression and faster improvement in its treatment process.
Stress
Many studies have indicated the role of genetic and environmental factors in depression. The major environmental factor that, directly and indirectly, leads to depression is stress [8, 13]. Accordingly, it is defined as the environmental changes, internal or external, that would disturb homeostasis maintenance in the body [22]. Environmental stressors could worsen the prognostic indicators of depression [23], as many patients with a stressful lifestyle also experienced MDD episodes [24] (fig. 4).Stress activates the HPA axis and sympathetic nervous system leading to the release of glucocorticoids and catecholamines into the blood [25]. Long exposure to glucocorticoids has neurotoxic effects on the brain [26]. Therefore, it could cause many neurological and psychological diseases similar to depression. Many studies have confirmed the relationship between depression and stress-related structural changes in the brain that alter mood and cause physical dysfunctions [4, 27, 28]. Therefore, chronic stress, which changes the body’s homeostasis, could eventually lead to depression or worsened symptoms of depression.
Gender
The clinical manifestation of depression was reported at higher levels among women [29]. Surprisingly, some studies have also suggested the role of genetic factors in depression in women [17]. Psychiatric disorders are also linked to gender differences, especially at the onset of puberty until approaching menopause in women [30]. Since women are more likely to be diagnosed with depression, careful attention to different factors, such as their hormonal changes, is required before and during the treatment process.Neural connections
Neural connections would be disrupted in depression [31]. Depression alters the organizational microstructure of white matter pathways in some brain regions, such as frontolimbic neural pathways [32], and might also be involved in neurode-generation and aberrant neural network functions [33] (fig. 5).
Figure 4. Stress, anxiety, and depression overlap. However, in the etiology of anxiety and depression, psychological stress is a major risk factor [54]

Figure 5. A conceptual scheme for the major brain network circuit involved in depression-related behaviors and neurotransmitter secretion from different brain nuclei [116]
Role of different brain structures in depression
Some neurobiological, structural, and functional abnormalities have been observed in several limbic [amygdala, hippocampus, and nucleus accumbens (NAc)], non-limbic, or para-limbic [cortical areas, subgenual anterior cingulate cortex (sACC), and ventromedial PFC (vmPFC)] structures that were involved in the prefrontal and cingulate cortices [8, 34] (fig. 6).Patients with MDD had a smaller volume of the medial orbitofrontal cortex (mOFC or gyrus rectus) [35]. The activity in the dorsal system [hippocampus, dorsolateral PFC (DL-PFC), and dorsal anterior cingulate cortex (dACC)] is reduced in depression. In similar conditions, increased activity has been observed in the ventral system (amygdala, insula, ventral striatum, subgenual cingulate cortex, ventral parts of the ACC and PFC) [34, 36].
In people with depression, morphological alterations, including neural atrophy, reduced number of glial cells, dendritic spines, brain metabolism, brain volume, and dendritic arborization have been reported in several cortical and limbic areas like PFC and hippocampus [8, 25, 37]. Similarly, some changes were observed in the gray matter volume, neural organization, electro-physiological activity, and neurotransmitter receptors in the mentioned brain regions [34]. As shown in Figure 7, despite the hyperactivation of some brain areas in depression, other regions may become hypoactive [38]. As such, several brain regions changed in patients with depression, including the limbic system, hippocampus,

Figure 6. Limbic regions have reciprocal connections with the “para-limbic” cortical areas, subgenual anterior cingulate, and vmPFC [117]

Figure 7. Some brain areas are hypoactive, whereas other regions are hyperactive in depression
amygdala, and PFC [39]. For instance, those patients who committed suicide had different connections between the left anterior limb of the internal capsule (ALIC), left middle frontal cortex (mFC), orbitofrontal cortex (OFC), and left thalamus [40]. Therefore, evaluating different parts of the brain via imaging techniques before, throughout, and after the treatment process could help estimate the treatment effect.
The ionotropic N-methyl-D-aspartate (NMDA) and amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are involved in the glutamate release [59]. The NMDA receptor antagonists act as potent and fast-acting antidepressants [56] by regulating moods, possibly through neuroplasticity maintenance [56], leading to the alleviation of depression-related symptoms [47]. Therefore, the pharmacologic stimulation of AMPA receptors facilitates depression recovery [47].
Due to the potential role of different neurotransmitters in provoking the occurrence of depression, it is necessary to use various drugs for the treatment of depression in such a way that they could affect different neurotransmitters, especially for resistant and severe types of depression.
According to the effects of different hormones on developing depression, a laboratory examination is necessary before initiating the treatment. In this regard, hormone therapy could simultaneously be considered along with other types of drugs. Since the functional level and blood maintenance of different hormones affect the body’s physiologic system more than the neurotransmitter secretion, the changes in neurotransmitter secretion in specific brain regions seem more accessible than the changes in hormone secretion.
less depressive-like behavior [33, 107, 109].
At last, the role of different brain areas, systems, and biochemical factors in depression are summarized in Figure 8.
Limbic region
The limbic system is a set of brain structures that collectively affect emotions, behavioral patterns, memory, and olfactory modality [41]. Any structural changes in this system would be associated with depression [8, 42].Hippocampus
The hippocampus, a significant part of the limbic system, is involved in the pathophysiology of depression [43]; for example, hippocampal volume was reported to have decreased in depression [44]. The changes in hippocampal function may also influence other limbic structures, including the PFC, amygdala, ventral tegmental area (VTA), and NAc, which are associated with the state of mood and emotions [45]. Moreover, depression causes memory impairment, possibly in association with decreased hippocampal plasticity [12, 46]. The hippocampal connections to key frontal and subcortical regions (amygdala, hypothalamus, basal ganglia, and PFC) indicate that the hippocampus is involved in regulating mood [41]. Consequently, any hippocampal dysfunctionality could result in inappropriate emotional responses [14].Amygdala
The amygdala is a brain area that processes threats and regulates emotions [47]; therefore, abnormal amygdala activity plays a role in the severity of depression [14]. Increased amygdala blood flow and metabolism were observed in subjects with depression [47]. The volume, glial density, and glia/neuron ratio were reduced in the amygdala of depressed patients [41, 48]. Chronic stress increased the spine density, synaptogenesis, and metabolism in the amygdala [49]. Moreover, serotonin transporters were significantly reduced in the amygdala of depressed patients [8].Para-limbic regions
Prefrontal cortex (frontolimbic)
The vmPFC controls the autonomic nervous system, cognition, and emotions [20]. Apart from a decreased activity in the DL-PFC, the ventral prefrontal and para-limbic structures presented increased activity in depression [47]. The depression rate had a reverse correlation with the left dorsal PFC activity [47]. Similarly, the ventrolateral (i.e., anterior cingulate) and orbital areas of the PFC had abnormal blood flow and metabolism in depression, where many interconnections were observed with the amygdala, dorsomedial nucleus of the thalamus, and ventral striatum (both ventromedial caudate and NAc) [50].Raphe nuclei
Dorsal and median raphe nuclei neurons are the primary serotonin sources in the forebrain [51]. Brain imaging studies have shown that serotonin transporters were decreased in the midbrain raphe nuclei in MDD [52].Other structures
Depression changes the volume of some brain regions, such as the thalamus and insula [53, 54]. For instance, a decreased size of the striatum and caudate nuclei was observed [41]; these areas also exhibited lower ventral striatum activity in depression [55]. Therefore, the limbic system seems more involved in depression than the para-limbic system.4. Neurobiology of depression
A vast range of neurobiological changes in the brain is associated with depression, including the release of neurotransmitters, hormones, inflammatory factors (cytokines), and neurotrophic factors [14, 56-58]. Some of these neurobiological factors are indicated in the present study, as will be observed in the following sections.Role of neurotransmitters in depression
Many neurotransmitters alterations affect the mood states and lead to depression [59] (see fig. 4). For instance, the changes in the amount of monoamines (i.e., serotonin, norepinephrine, and dopamine) lead to depression [37, 56, 60]. Additionally, the down-regulation and desensitization processes of pre- and post-synaptic norepinephrine and serotonin receptors change in depression [37].Serotonin
Serotonin plays a vital role in various brain functions, including mood, anxiety, aggression, sleeping habits, appetite, sexual desire, and especially learning and memory [61, 62]; that is because serotonergic neurotransmission deficits are linked to depression [44]. According to some reports, reduced serotonin levels were not observed in all depressed patients [63]. Neurogenesis was decreased with lower serotonin levels in the dorsal raphe of the rodents in depression [56]. Furthermore, reduced serotonin neurotransmission was observed in most forebrain regions, including the medulla oblongata, raphe nucleus, frontal cortex, and hippocampus [8, 64]. However, the serotonergic activity enhanced in pons in similar conditions [64]. The impaired serotonin input to other brain areas, including DL-PFC, subgenual PFC, and amygdala, could lead to mood impairment and depression [65]. In addition, significant changes in neural activity were observed in several serotonin-related brain regions (e.g., dorsal raphe, septal region, habenula, amygdala, and OFC), indicating that tryptophan plasma levels were related to depressed moods [47]. Various studies on the effects of serotonin reuptake inhibitors administration have confirmed the role of serotonin in alleviating some depression symptoms [11].Norepinephrine
Several studies proved the involvement of low concentrations of plasma norepinephrine in depression [60]. The neural response occurs via up-regulation of the postsynaptic receptors to reduce monoamine transmission as a compensatory mechanism for depression [66]. It is notable that most therapeutic drugs for depression target monoaminergic systems [67].Dopamine
Dopamine is the major mediator of the pleasure and reward system [47] and it is indicated as a motivational factor of behavior [39]. In the mesolimbic pathway, as a key reward circuit, the dopaminergic neurons project from VTA to NAc, hippocampus, PFC, and other forebrain areas [47, 56, 68]. These dopaminergic neurons also play a crucial role in mediating stress response, which is the main MDD cause [69].Glutamate
Glutamate is a major excitatory neurotransmitter in the nervous system [64, 70] and is responsible for regulating mood states [56]. Moreover, it is essential for dendritic development and neural growth [70]. The glial cells (i.e., astrocytes, oligodendrocytes, and microglia) have a key role in regulating glutamate signaling in depression [71, 72]. Notably, depression is associated with reduced glutamate function in the prefrontal regions [71].The ionotropic N-methyl-D-aspartate (NMDA) and amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors are involved in the glutamate release [59]. The NMDA receptor antagonists act as potent and fast-acting antidepressants [56] by regulating moods, possibly through neuroplasticity maintenance [56], leading to the alleviation of depression-related symptoms [47]. Therefore, the pharmacologic stimulation of AMPA receptors facilitates depression recovery [47].
Gamma-aminobutyric acid
Gamma-aminobutyric acid (GABA) is implicated in depression [25]. Previous studies have frequently mentioned the varying GABA levels in the brain of patients with depression [73]. Anxiety and depressive disorders seem to deregulate GABAergic signaling [64], GABA transporter expression, and enzyme involvement of GABA metabolism in depression [13].Substance P
Substance P is pathologically involved in depression [74, 75], where levels of serum substance P are enhanced [76]. However, its antagonists had no antidepressive effects [77].Due to the potential role of different neurotransmitters in provoking the occurrence of depression, it is necessary to use various drugs for the treatment of depression in such a way that they could affect different neurotransmitters, especially for resistant and severe types of depression.
Role of hormones in depression
According to previous studies, depression is associated with changes in neurotransmitters. However, the alteration of some hormone circadian rhythms leads to depression as well [36].Glucocorticoids
Abnormal activation of the HPA axis is implicated in depression [25, 78]. Glucocorticoids could contribute to depression pathogenesis by decreasing synaptic plasticity and augmenting vulnerability to neural death in the hippocampus [79]. Several factors, including monoamine dysfunction, neurogenesis decrease, synaptic neuroplasticity, increased neurodegeneration, and various changes in different brain regions, are linked to depression. These factors are associated with the Glucocorticoid Theory of Depression [37].Thyroid hormone
There is a link between thyroid disorders and depression [80], as thyroid hormone concentrations are involved in the severity of depressive episodes [81]. For instance, although hypothyroidism decreases anxiety and depression-like behaviors, an opposite result was observed in the hyperthyroid rats [82]. As a treatment strategy for depression, levothyroxine could be used [83]. Although some studies reported levothyroxine to have improved mood without causing hyperthyroid symptoms in depressive subjects [84], other studies showed no significant relationship between thyroid disorders and depression [85, 86]. Nevertheless, there is still insufficient evidence to support thyroid hormones as a suitable treatment strategy for unipolar depression [87].Estrogen
Ample evidence exists for the high incidence of depression among women due to serum estrogen and even progesterone levels [88, 89]. However, menopause, pregnancy, menses, and fluctuating hormones increase the occurrence rate of depressed mood states in women [89, 90]. As such, estrogen therapy improves the mood of women after menopause [4]. Serotonin and noradrenaline levels were enhanced after menopause, and estrogen participated in regulating the serotonin receptors in the brain [89, 91]. It is noteworthy that estrogen increases serotonin levels by enhancing serotonin synthesis and decreasing its reuptake [89].Testosterone
The relationship between depression and serum testosterone levels remains unclear. A weak association between testosterone concentrations and depression has been observed [92]. However, it should be mentioned that salivary testosterone levels have been lower in patients with depression [93].Parathyroid hormone
Some patients with hyperparathyroidism exhibited mood swings [94]. It is reported that vitamin D deficiency increases serum parathyroid hormone (PTH) levels, a hormone that is commonly accompanied by depression [95]. Some other studies suggested no association between the levels of PTH and vitamin D in depression [96].Oxytocin and antidiuretic hormone
Oxytocin seems to be involved in regulating anxiety and the HPA axis. An increase in the plasma levels of oxytocin in depression has been reported [97]. Similarly, antidiuretic hormone levels increased in a hyperactive HPA axis in depression conditions [98, 99].Melatonin
Melatonin is involved in regulating the circadian rhythms and biological clock. A disturbed circadian rhythm could increase the risk of depression [100], and nocturnal concentrations of melatonin have been higher in patients with MDD [101].Insulin
Insulin increases serotonin in the brain through different mechanisms, including enhanced serotonin synthesis rate and tryptophan entry into the brain, thus, affecting mood states in depression [102].According to the effects of different hormones on developing depression, a laboratory examination is necessary before initiating the treatment. In this regard, hormone therapy could simultaneously be considered along with other types of drugs. Since the functional level and blood maintenance of different hormones affect the body’s physiologic system more than the neurotransmitter secretion, the changes in neurotransmitter secretion in specific brain regions seem more accessible than the changes in hormone secretion.
Role of other biochemical factors in depression
Inflammatory factors (Cytokines)
An elevated level of circulating immune markers (e.g., cytokines) has an immunological function, and is also important for the formation of neural structures and circuits [8]. Cytokines cause major changes in systems relevant to the development of depression, such as the HPA axis and sympathetic nervous system [37]. Therefore, the immune system has a critical role in neurodevelopment [8], as depressed patients exhibit an increase in autoimmune disorders [56]. Cytokines are divided into different categories: interleukins (pro- and anti-inflammatory ILs), chemokines, tumor necrosis factors (TNFs, TNFα), interferons (IFNs, IFNα), and transforming growth factors (TGFs, TGF-β) [37]. The overexpression of pro-inflammatory cytokines is reported in the brain of many patients with depression [56, 79, 103]. There is also a strong association between depression and peripheral inflammatory diseases [104]. The inflammatory cytokines reduced monoamine levels in depressed patients through increasing tryptophan metabolism [56]. The activation of the immune system and neuro-inflammation could cause a deficiency in the dopaminergic mesolimbic pathway and dysfunction in the prefrontal glutamatergic system. This leads to anhedonia, loss of motivation, psychomotor retardation, fatigue, and cognitive deficits [79, 105]. Some cytokines (like IL-6) modulate neuro-transmission and activate the HPA axis leading to depression [106]. Moreover, plasma levels of anti-inflammatory cytokines were reduced in depression [79]. The patients under cytokine immunotherapy, for instance, those receiving IFNα, presented a higher risk of developing depression-related symptoms [25].Neurotrophic factors
Brain-derived neurotrophic factor (BDNF) and other members of the neurotrophic factor family, including the neurotrophin-3 and nerve growth factor, affect cellular functions [107, 108], which seem to be linked to the clinical manifestations of depression [55]. In addition, BDNF has a crucial role in the neurobiology of depression [8, 79]. It regulates neural morphology and physiology, and structural complexities [107]. In several studies, reduced BDNF levels were reported in MDD patients [35, 56]. However, their over-expression in the forebrain excitatory neurons of the hippocampus, neocortex, and amygdala was seen withless depressive-like behavior [33, 107, 109].
Brain functions in depression
Neuroplasticity
Depression is associated with changes in neuroplasticity, neurogenesis, and neurotransmitters in certain corticolimbic structures, such as the hippocampus and PFC [25, 107]. Neuro-psychological studies indicate that cognitive impairments are related to the frontal lobe functions in depression [52].Reward system
Rewards could motivate the recipient to obtain survival necessities [110]. The VTA, NAc, midbrain, vmPFC, posterior cingulate cortex, ACC, anterior insula, and thalamus are involved in the reward system, whose alterations could generate anhedonia [56, 111] as one of the depression-related symptoms [93]. According to some studies, dopaminergic neurotransmission was diminished in depression [112].Behavioral patterns
Depression has several psychological, behavioral, and physiological symptoms. These symptoms include dark moods, negative thinking, cognitive dysfunctions, irritability, sadness, social isolation, insomnia, fatigue, impaired concentration, low self-esteem, guilt, crying uncontrollably, appetite disturbance, constipation, weight fluctuation, libido loss, sexual dysfunction, lack of energy, motivation and memory impairment, slower speech, anhedonia, feelings of worthlessness, and finally suicidal thoughts [37, 56, 103]; which is why it is associated with higher rates of morbidity, mortality, and increased risk of suicide as well [14, 113].At last, the role of different brain areas, systems, and biochemical factors in depression are summarized in Figure 8.

Figure 8. A summary diagram of how depression affects different brain aspects
Conclusions
Depression is a mental disorder caused by biochemical and morphological alterations in different brain areas, in which the limbic system is more involved than the para-limbic one. Changes in neurotransmitter secretion in specific brain regions also seem to be more accessible compared to hormone secretion fluctuations. Overall, a combination of different genetic, environmental, and epigenetic factors are involved in the development of this disorder. The genetic and epigenetic modifications would require high costs. Therefore, it seems that the best available strategy for depression treatment is making changes in environmental factors, which could alter the secretion of brain neurotransmitters and different hormones. As a result, an alternative lifestyle with exercise, balanced nutrition, and the use of medications with low side effects are proposed as the best approach to depression treatment. However, making a proper decision for the best treatment scheme needs comprehensive knowledge of the physiopathology of depression. A therapist should therefore investigate all the underlying factors through various tests before prescribing different drugs. At last, it is strongly recommended to avoid one-dimensional treatment procedures and consider all the influencing factors in depression for a more effective treatment strategy.
Compliance with ethical guidelines
All ethical principles were considered in conducting the present study.
Acknowledgments
The authors would like to express their gratitude to Isfahan University of Medical Sciences, Isfahan, Iran. This research has received no grant from any funding agency, charitable or non-charitable institutions. All authors contributed to the literature search, analysis of the published data, and writing and revision of the manuscript. Hence, no conflict of interest is declared regarding this research.
Authorsʼ contributions
All authors participated in drafting of the article and approved the final version.
Funding/Support
None.
Conflicts of Interest
The authors declare no conflict of interests.
References
1. Kendler K, Halberstadt L. The road not taken: life experiences in monozygotic twin pairs discordant for major depression. Molecular Psychiatry. 2013; 18(9):975-84. [DOI:10.1038/mp.2012.55] [PMID] [PMCID]
2. Baskaran A, Milev R, McIntyre RS. The neurobiology of the EEG biomarker as a predictor of treatment response in depression. Neuropharmacology. 2012; 63(4):507-13. [DOI:10.1016/j.neuropharm.2012.04.021] [PMID]
3. Reiss K, Rhodes EF. Translation criticism–the potentials and limitations: Categories and criteria for translation quality assessment. Routledge; 2014.
4. McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005; 54(5):20-3. [DOI:10.1016/j.metabol.2005.01.008] [PMID]
5. Zamani M, Radahmadi M, Reisi P. Therapeutic effects of exercise-accompanied escitalopram on synaptic potency and long-term plasticity in the hippocampal CA1 area in rats under chronic restraint stress. Iranian Journal of Basic Medical Sciences. 2022; 25(12). [DOI:10.22038/IJBMS.
2022.66718.14629] [PMID] [PMCID]
6. Ostinelli EG, Zangani C, Giordano B, Maestri D, Gambini O, D’Agostino A, et al. Depressive symptoms and depression in individuals with internet gaming disorder: A systematic review and meta-analysis. Journal of Affective Disorders. 2021; 284:136-42. [DOI:10.1016/j.jad.2021.
02.014] [PMID]
7. Dudek KA, Dion‐Albert L, Kaufmann FN, Tuck E, Lebel M, Menard C. Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. European Journal of Neuroscience. 2021; 53(1):183-221. [DOI:10.1111/ejn.14547] [PMID] [PMCID]
8. Lima-Ojeda JM, Rupprecht R, Baghai TC. Neurobiology of depression: a neurodevelopmental approach. The World Journal of Biological Psychiatry. 2018; 19(5):349-59. [DOI:10.1080/15622975.2017.1289240] [PMID]
9. Linde K, Rücker G, Sigterman K, Jamil S, Meissner K, Schneider A, et al. Comparative effectiveness of psychological treatments for depressive disorders in primary care: network meta-analysis. BMC Family Practice. 2015; 16(1):1-14. [DOI:10.1186/s12875-015-0314-x] [PMID] [PMCID]
10. Young N. Non-pharmacological treatments for patients with depression. Nursing Standard. 2013; 28(7):43-51. [DOI:10.
7748/ns2013.10.28.7.43.e7577]
11. Zamani M, Radahmadi M, Reisi P. Therapeutic effects of exercise, escitalopram and exercise-accompanied escitalopram on brain functions in rats with depression. Physiology and Pharmacology. 2022; 26(2):188-99. [DOI:10.52547/phypha.26.2.7]
12. Joodaki M, Radahmadi M, Alaei H. Comparing the Therapeutic Effects of Crocin, Escitalopram and Co-Administration of Escitalopram and Crocin on Learning and Memory in Rats with Stress-Induced Depression. The Malaysian Journal of Medical Sciences. 2021; 28(4):50. [DOI:10.21315/mjms2021.28.4.6] [PMID] [PMCID]
13. Fiori LM, Turecki G. Broadening our horizons: gene expression profiling to help better understand the neurobiology of suicide and depression. Neurobiology of Disease. 2012; 45(1):14-22. [DOI:10.1016/j.nbd.2010.
11.004] [PMID]
14. Palazidou E. The neurobiology of depression. British Medical Bulletin. 2012; 101(1):127-45. [DOI:10.1093/bmb/
lds004] [PMID]
15. Zalsman G, Huang Y-y, Oquendo MA, Burke AK, Hu X-z, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006; 163(9):1588-93. [DOI:10.1176/ajp.2006.163.9.1588] [PMID]
16. Skoog I, Waern M, Duberstein P, Blennow K, Zetterberg H, Börjesson-Hanson A, et al. A 9-year prospective population-based study on the association between the APOE* E4 allele and late-life depression in Sweden. Biological Psychiatry. 2015; 78(10):730-6. [DOI:10.1016/j.biopsych.2015.01.006] [PMID]
17. Kang H-J, Park Y, Yoo K-H, Kim K-T, Kim E-S, Kim J-W, et al. Sex differences in the genetic architecture of depression. Scientific Reports. 2020; 10(1):1-12. [DOI:10.1038/s41598-020-66672-9] [PMID]
18. Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience. 2008; 28(36):9055-65. [DOI:10.1523/
JNEUROSCI.1424-08.2008] [PMID] [PMCID]
19. Verduijn J, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman AT, Penninx BW. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Translational Psychiatry. 2015; 5(9):e649-e. [DOI:10.1038/tp.2015.137] [PMID] [PMCID]
20. Agelink MW, Klimke A, Cordes J, Sanner D, Kavuk I, Malessa R, et al. A functional-structural model to understand cardiac autonomic nervous system (ANS) dysregulation in affective illness and to elucidate the ANS effects of antidepressive treatment. European Journal of Medical Research. 2004; 9(1):37-50. [PMID]
21. Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B12 deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. American Journal of Psychiatry. 2000; 157(5):715-21. [DOI:10.1176/
appi.ajp.157.5.715] [PMID]
22. Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. European Psychiatry. 2005; 20:S302-S6. [DOI:10.1016/s0924-9338
(05)80180-4] [PMID]
23. Gilman SE, Trinh N-H, Smoller JW, Fava M, Murphy JM, Breslau J. Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychological Medicine. 2013; 43(2):303-16. [DOI:10.1017/S0033291712001080] [PMID]
24. Cohen S, Murphy ML, Prather AA. Ten surprising facts about stressful life events and disease risk. Annual review of psychology. 2019; 70:577. [DOI:10.1146/annurev-psych-010418-102857] [PMID]
25. Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nature Reviews Neuroscience. 2016; 17(8):497. [DOI:10.1038/nrn.2016.69] [PMID] [PMCID]
26. Li J, Chen J, Ma N, Yan D, Wang Y, Zhao X, et al. Effects of corticosterone on the expression of mature brain-derived neurotrophic factor (mBDNF) and proBDNF in the hippocampal dentate gyrus. Behavioural Brain Research. 2019; 365:150-6. [DOI:10.1016/j.bbr.2019.03.010] [PMID]
27. Sgoifo A, Carnevali L, Pico Alfonso MdlA, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015; 18(3):343-52. [DOI:10.3109/102
53890.2015.1045868] [PMID]
28. Murray G. Diurnal mood variation in depression: a signal of disturbed circadian function? Journal of affective Disorders. 2007; 102(1-3):47-53. [DOI:10.1016/j.jad.2006.12.001] [PMID]
29. Ma L, Xu Y, Wang G, Li R. What do we know about sex differences in depression: a review of animal models and potential mechanisms. Progress in Neuro-Psycho-pharmacology and Biological Psychiatry. 2019; 89:48-56. [DOI:10.1016/j.pnpbp.2018.08.026] [PMID]
30. Van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ, Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011; 191:38-45. [DOI:10.1016/j.neuroscience.2011.04.042] [PMID]
31. Long Z, Duan X, Wang Y, Liu F, Zeng L, Zhao J-p, et al. Disrupted structural connectivity network in treatment-naive depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015; 56:18-26. [DOI:10.1016/j.
pnpbp.2014.07.007] [PMID]
32. Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010; 49(2):173-83. e1. [DOI:10.1097/00004583-201002000-00011] [PMID] [PMCID]
33. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Developmental neurobiology. 2010; 70(5):289-97. [DOI:10.1002/dneu.20758] [PMID]
34. Castrén E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 2013; 70(9):983-9. [DOI:10.
1001/jamapsychiatry.2013.1] [PMID]
35. Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002; 51(4):273-9. [DOI:10.1016/s0006-3223(01)01336-1] [PMID]
36. Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry. 2014; 76(3):258-66. [DOI:
10.1016/j.biopsych.2013.11.027] [PMID]
37. Boas GRV, de Lacerda RB, Paes MM, Gubert P, da Cruz Almeida WL, Rescia VC, et al. Molecular aspects of depression: a review from neurobiology to treatment. European Journal of Pharmacology. 2019; 851:99-121. [DOI:10.1016/j.ejphar.2019.02.024] [PMID]
38. Palmer SM, Crewther SG, Carey LM. A meta-analysis of changes in brain activity in clinical depression. Frontiers in Human Neuroscience. 2015; 8:1045. [DOI:10.3389/
fnhum.2014.01045]
39. Kern N, Sheldrick AJ, Schmidt FM, Minkwitz J. Neurobiology of depression and novel antidepressant drug targets. Current Pharmaceutical Design. 2012; 18(36):5791-801. [DOI:10.2174/138161212803523581] [PMID]
40. Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: Psychoradiological evidence. CNS Neuroscience & Therapeutics. 2018; 24(11):
994-1003. [DOI:10.1111/cns.12835] [PMID]
41. Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in Neurobiology. 2012; 98(1):99-143. [DOI:10.1016/j.pneurobio.2012.05.009] [PMID]
42. Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—the SMART Medea study. Biological psychiatry. 2011; 70(4):373-80. [DOI:10.1016/j.biopsych.
2011.01.029] [PMID]
43. Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, et al. Hippocampal changes in patients with a first episode of major depression. American Journal of Psychiatry. 2002; 159(7):1112-8. [DOI:10.1176/appi.ajp.
159.7.1112] [PMID]
44. Jain N, Steffens DC. Neurobiology and risk factors of late-life depression. Understanding Depression. Springer; 2018.
45. Thakker-Varia S, Alder J. Neuropeptides in depression: role of VGF. Behavioural Brain Research. 2009; 197(2):262-78. [DOI:10.1016/j.bbr.2008.10.006] [PMID] [PMCID]
46. Fossati P, Radtchenko A, Boyer P. Neuroplasticity: from MRI to depressive symptoms. European Neuropsycho-pharmacology. 2004; 14:S503-S10. [DOI:10.1016/j.euro
neuro.2004.09.001] [PMID]
47. Gold PW, Machado-Vieira R, Pavlatou MG. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural plasticity. 2015; 2015:581976. [DOI:10.1155/2015/581976] [PMID] [PMCID]
48. Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002; 52(5):404-12. [DOI:10.1016/
s0006-3223(02)01404-x] [PMID]
49. Huang P, Gao T, Dong Z, Zhou C, Lai Y, Pan T, et al. Neural circuitry among connecting the hippocampus, prefrontal cortex and basolateral amygdala in a mouse depression model: associations correlations between BDNF levels and BOLD–fMRI signals. Brain Research Bulletin. 2018; 142:107-15. [DOI:10.1016/j.brainresbull.2018.06.
019] [PMID]
50. Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual review of medicine. 1998; 49(1):341-61. [DOI:10.1146/annurev.med.49.1.341] [PMID]
51. Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsycho-pharmacology. 2006; 31(4):814-24. [DOI:10.1038/sj.npp.
1300897] [PMID]
52. Bhagya V, Srikumar B, Raju T, Rao BS. Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology. 2011; 214(2):477-94. [DOI:10.1007/s00213-010-2054-x] [PMID]
53. Nugent AC, Davis RM, Zarate Jr CA, Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry Research: Neuroimaging. 2013; 213(3):179-85. [DOI:10.
1016/j.pscychresns.2013.05.004] [PMID]
54. Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, et al. The insular cortex and the neuroanatomy of major depression. Journal of Affective Disorders. 2011; 133(1-2):120-7. [DOI:10.1016/j.jad.
2011.04.004] [PMID] [PMCID]
55. Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neuroscience & Biobehavioral Reviews. 2013; 37(10):2331-71. [DOI:
10.1016/j.neubiorev.2012.12.007] [PMID]
56. Dean J, Keshavan M. The neurobiology of depression: An integrated view. Asian journal of psychiatry. 2017; 27:101-11. [DOI:10.1016/j.ajp.2017.01.025] [PMID]
57. Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Translational psychiatry. 2012; 2(2):e79-e. [DOI:10.1038/tp.2012.8] [PMID] [PMCID]
58. Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010; 34(5):766-76. [DOI:10.1016/j.pnpbp.
2009.09.006] [PMID]
59. Pitsillou E, Bresnehan SM, Kagarakis EA, Wijoyo SJ, Liang J, Hung A, et al. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Molecular Biology Reports. 2020; 47(1):753-70. [DOI:10.1007/s11033-019-05129-3] [PMID]
60. Ferrari F, Villa R. The neurobiology of depression: an integrated overview from biological theories to clinical evidence. Molecular Neurobiology. 2017; 54(7):4847-65. [DOI:10.1007/s12035-016-0032-y] [PMID]
61. Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. The British Journal of Psychiatry. 2001; 178(5):399-405. [DOI:10.1192/bjp.178.5.399] [PMID]
62. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Reviews in the Neurosciences. 2012; 23(5-6):543-53. [DOI:10.1515/revneuro-2012-0060] [PMID]
63. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opinion on Therapeutic Targets. 2011; 15(4):379-400. [DOI:10.1517/14728222.2011.553603] [PMID]
64. Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neuroscience & Biobehavioral Reviews. 2012; 36(9):2085-117. [DOI:10.1016/j.neubiorev.2012.07.001] [PMID] [PMID]
65. Mann J, Currier D. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. European Psychiatry. 2010; 25(5):268-71. [DOI:10.1016/j.
eurpsy.2010.01.009] [PMID] [PMCID]
66. Chen MJ. The neurobiology of depression and physical exercise. InRoutledge handbook of physical activity and mental health: Routledge; 2013.
67. Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Research Reviews. 2009; 61(2):105-23. [DOI:10.1016/j.
brainresrev.2009.05.005] [PMID]
68. Cathomas F, Murrough JW, Nestler EJ, Han M-H, Russo SJ. Neurobiology of resilience: interface between mind and body. Biological Psychiatry. 2019; 86(6):410-20. [DOI:10.
1016/j.biopsych.2019.04.011] [PMID]
69. Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013; 493(7433):532-6. [DOI:10.1038/nature11713] [PMID] [PMCID]
70. Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Therapeutic Advances in Chronic Disease. 2015; 6(3):97-114. [DOI:10.1177/2040
622315579059] [PMID] [PMCID]
71. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011; 35(3):537-55. [DOI:10.1016/j.neubiorev.2010.06.006] [PMID] [PMCID]
72. Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nature Reviews Drug Discovery. 2017; 16(7):472-86. [DOI:10.1038/nrd.2017.16] [PMID]
73. Duman RS, Shinohara R, Fogaça MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Molecular psychiatry. 2019:1-17. [DOI: 10.1038/s41380-019-0400-x] [PMID] [PMCID]
74. Frisch P, Bilkei-Gorzó A, Rácz I, Zimmer A. Modulation of the CRH system by substance P/NKA in an animal model of depression. Behavioural Brain Research. 2010; 213(1):103-8. [DOI:10.1016/j.bbr.2010.04.044] [PMID]
75. Ranga K, Krishnan R. Clinical experience with substance P receptor (NK1) antagonists in depression. The Journal of Clinical Psychiatry. 2002; 63:25. [PMID]
76. Bondy B, Baghai TC, Minov C, Schüle C, Schwarz MJ, Zwanzger P, et al. Substance P serum levels are increased in major depression: preliminary results. Biological Psychiatry. 2003; 53(6):538-42. [DOI:10.1016/s0006-3223(02)01544-5] [PMID]
77. Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, et al. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological Psychiatry. 2006; 59(3):216-23. [DOI:10.1016/j.biopsych.2005.07.013] [PMID]
78. Narasimhan M, Campbell N. A tale of two comorbidities: understanding the neurobiology of depression and pain. Indian Journal of Psychiatry. 2010; 52(2):127. [DOI:10.4103/0019-5545.64586] [PMID]
79. Caraci F, Calabrese F, Molteni R, Bartova L, Dold M, Leggio GM, et al. International :union: of basic and clinical pharmacology CIV: The neurobiology of treatment-resistant depression: From antidepressant classifications to novel pharmacological targets. Pharmacological Reviews. 2018; 70(3):475-504. [DOI:10.1124/pr.117.014977] [PMID]
80. Ittermann T, Völzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Social Psychiatry and Psychiatric Epidemiology. 2015; 50(9):1417-25. [DOI:10.1007/s00127-015-1043-0] [PMID]
81. Berent D, Zboralski K, Orzechowska A, Gałecki P. Thyroid hormones association with depression severity and clinical outcome in patients with major depressive disorder. Molecular Biology Reports. 2014; 41(4):2419-25. [DOI:10.1007/s11033-014-3097-6] [PMID] [PMCID]
82. Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, et al. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety-and depression-like behaviors in rats. Hormones and Behavior. 2015; 69:106-15. [DOI:10.1016/j.yhbeh.
2015.01.003] [PMID]
83. Bauer M, Whybrow P. Role of thyroid hormone therapy in depressive disorders. Journal of Endocrinological Investigation. 2021; 44(11):2341-7. [DOI:10.1007/s40618-021-01600-w] [PMID] [PMCID]
84. Moon JH, Han JW, Oh TJ, Choi SH, Lim S, Kim KW, et al. Effect of increased levothyroxine dose on depressive mood in older adults undergoing thyroid hormone replacement therapy. Clinical Endocrinology. 2020; 93(2):196-203. [DOI:10.1111/cen.14189] [PMID]
85. Almeida OP, Alfonso H, Flicker L, Hankey G, Chubb SP, Yeap BB. Thyroid hormones and depression: the Health in Men study. The American Journal of Geriatric Psychiatry. 2011; 19(9):763-70. [DOI:10.1097/JGP.0b013e31820dca
d5] [PMID]
86. Engum A, Bjøro T, Mykletun A, Dahl AA. An association between depression, anxiety and thyroid function–a clinical fact or an artefact? Acta Psychiatrica Scandinavica. 2002; 106(1):27-34. [DOI:10.1034/j.1600-0447.2002.01250.x] [PMID]
87. Lorentzen R, Kjaer JN, Østergaard S, Madsen M. Thyroid hormone treatment in the management of treatment‐resistant unipolar depression: a systematic review and meta‐analysis. Acta Psychiatrica Scandinavica. 2020; 141(4):316-26. [DOI:10.1111/acps.13154] [PMID]
88. Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric Disease and treatment. 2011; 7:9-13. [DOI:10.2147/NDT.S19619] [PMID] [PMCID]
89. Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. Journal of affective disorders. 2003; 74(1):85-96. [DOI:10.1016/s0165-0327(02)00428-7] [PMID] [PMCID]
90. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006; 63(4):375-82. [DOI:10.1001/archpsyc.
63.4.375] [PMID]
91. Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depression and Anxiety. 2007; 24(7):495-517. [DOI:10.1002/da.20262] [PMID]
92. Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Archives of General Psychiatry. 2008; 65(3):283-9. [DOI:10.1001/archgenpsychiatry.2007.33] [PMID]
93. Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008; 33(2):368-77. [DOI:10.1038/sj.npp.1301408] [PMID]
94. Espiritu RP, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA. Depression in primary hyperparathyroidism: prevalence and benefit of surgery. The Journal of Clinical Endocrinology & Metabolism. 2011; 96(11):E1737-E45. [DOI:10.1210/jc.
2011-1486] [PMID]
95. Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, et al. Learning as a model for neural plasticity in major depression. Biological Psychiatry. 2010; 68(6):544-52. [DOI:10.1016/j.biopsych.2010.05.026] [PMID]
96. Zhao G, Ford ES, Li C, Balluz LS. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. British Journal of Nutrition. 2010; 104(11):1696-702. [DOI:10.1017/S0007114510002588] [PMID]
97. Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, et al. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research. 2010; 178(2):359-62. [DOI:10.1016/j.psychres.2009.09.
017] [PMID]
98. Müller MB, Landgraf R, Keck ME. Vasopressin, major depression, and hypothalamic–pituitary–adrenocortical desensitization. Biological Psychiatry. 2000; 48(4):330-3. [DOI:10.1016/s0006-3223(00)00886-6] [PMID]
99. Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: a preliminary report. Biological Psychiatry. 2006; 60(8):892-5. [DOI:10.1016/j.biopsych.2005.12.010] [PMID]
100. Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, et al. Melatonin in mood disorders. The World Journal of Biological Psychiatry. 2006; 7(3):138-51. [DOI:10.1080/15622970600571822] [PMID]
101. Szymanska A, Rabe-Jablonska J, Karasek M. Diurnal profile of melatonin concentrations in patients with major depression: relationship to the clinical manifestation and antidepressant treatment. Neuroendocrinology Letters. 2001; 22(3):192-8. [PMID]
102. Zou XH, Sun LH, Yang W, Li BJ, Cui RJ. Potential role of insulin on the pathogenesis of depression. Cell Proliferation. 2020; 53(5):e12806. [DOI:10.1111/cpr.
12806] [PMID] [PMCID]
103. Dudek KA, Dion‐Albert L, Kaufmann FN, Tuck E, Lebel M, Menard C. Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. European Journal of Neuroscience. 2021; 53(1):183-221. [DOI:10.1111/ejn.14547] [PMID] [PMCID]
104. Na K-S, Lee KJ, Lee JS, Cho YS, Jung H-Y. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2014; 48:79-85. [DOI:10.1016/j.pnpbp.2013.09.006] [PMID]
105. Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry. 2010; 68(8):748-54. [DOI:10.1016/
j.biopsych.2010.06.010] [PMID] [PMCID]
106. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010; 67(5):446-57. [DOI:10.1016/j.biopsych.2009.09.033] [PMID]
107. Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Current Pharmaceutical Design. 2017; 23(21):3154-63. [DOI:10.2174/1381612823666170111141915] [PMID]
108. Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. Journal of Neuroscience. 2002; 22(8):3251-61. [DOI:10.1523/JNEUROSCI.22-08-03251.2002] [PMID] [PMCID]
109. Jaggar M, Fanibunda SE, Ghosh S, Duman RS, Vaidya VA. The neurotrophic hypothesis of depression revisited: new insights and therapeutic implications. Neurobiology of Depression: Elsevier; 2019. [DOI:10.1016/B978-0-12-813333-0.00006-8]
110. Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry. 2018; 175(11):1111-20. [DOI:10.1176/appi.ajp.2018.
17101124] [PMID] [PMCID]
111. Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015; 40(9):2258-68. [DOI:10.1038/npp.2015.75] [PMID] [PMCID]
112. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007; 64(3):327-37. [DOI:10.1001/archpsyc.
64.3.327] [PMID]
113. Korte SM, Prins J, Krajnc AM, Hendriksen H, Oosting RS, Westphal KG, et al. The many different faces of major depression: it is time for personalized medicine. European journal of Pharmacology. 2015; 753:88-104. [DOI:10.
1016/j.ejphar.2014.11.045] [PMID]
114. Cytokines and Depression: How Your Immune System Causes Depression. Ronald S Smith. Kindle Edition;1997.
115. Cohen-Woods S, Harkess KN. Gene-environment interactions, stress, and depression. 2016.
116. Liu H, Chaudhury D. Understanding mood disorders using electrophysiology and circuit breaking. decoding neural circuit structure and function: Springer; 2017.
117. Maletic V, Robinson M, Oakes T, Iyengar S, Ball S, Russell J. Neurobiology of depression: an integrated view of key findings. International Journal of Clinical Practice. 2007; 61(12):2030-40. [DOI:10.1111/j.1742-1241.2007.01602.x] [PMID] [PMCID]
Depression is a mental disorder caused by biochemical and morphological alterations in different brain areas, in which the limbic system is more involved than the para-limbic one. Changes in neurotransmitter secretion in specific brain regions also seem to be more accessible compared to hormone secretion fluctuations. Overall, a combination of different genetic, environmental, and epigenetic factors are involved in the development of this disorder. The genetic and epigenetic modifications would require high costs. Therefore, it seems that the best available strategy for depression treatment is making changes in environmental factors, which could alter the secretion of brain neurotransmitters and different hormones. As a result, an alternative lifestyle with exercise, balanced nutrition, and the use of medications with low side effects are proposed as the best approach to depression treatment. However, making a proper decision for the best treatment scheme needs comprehensive knowledge of the physiopathology of depression. A therapist should therefore investigate all the underlying factors through various tests before prescribing different drugs. At last, it is strongly recommended to avoid one-dimensional treatment procedures and consider all the influencing factors in depression for a more effective treatment strategy.
Compliance with ethical guidelines
All ethical principles were considered in conducting the present study.
Acknowledgments
The authors would like to express their gratitude to Isfahan University of Medical Sciences, Isfahan, Iran. This research has received no grant from any funding agency, charitable or non-charitable institutions. All authors contributed to the literature search, analysis of the published data, and writing and revision of the manuscript. Hence, no conflict of interest is declared regarding this research.
Authorsʼ contributions
All authors participated in drafting of the article and approved the final version.
Funding/Support
None.
Conflicts of Interest
The authors declare no conflict of interests.
References
1. Kendler K, Halberstadt L. The road not taken: life experiences in monozygotic twin pairs discordant for major depression. Molecular Psychiatry. 2013; 18(9):975-84. [DOI:10.1038/mp.2012.55] [PMID] [PMCID]
2. Baskaran A, Milev R, McIntyre RS. The neurobiology of the EEG biomarker as a predictor of treatment response in depression. Neuropharmacology. 2012; 63(4):507-13. [DOI:10.1016/j.neuropharm.2012.04.021] [PMID]
3. Reiss K, Rhodes EF. Translation criticism–the potentials and limitations: Categories and criteria for translation quality assessment. Routledge; 2014.
4. McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005; 54(5):20-3. [DOI:10.1016/j.metabol.2005.01.008] [PMID]
5. Zamani M, Radahmadi M, Reisi P. Therapeutic effects of exercise-accompanied escitalopram on synaptic potency and long-term plasticity in the hippocampal CA1 area in rats under chronic restraint stress. Iranian Journal of Basic Medical Sciences. 2022; 25(12). [DOI:10.22038/IJBMS.
2022.66718.14629] [PMID] [PMCID]
6. Ostinelli EG, Zangani C, Giordano B, Maestri D, Gambini O, D’Agostino A, et al. Depressive symptoms and depression in individuals with internet gaming disorder: A systematic review and meta-analysis. Journal of Affective Disorders. 2021; 284:136-42. [DOI:10.1016/j.jad.2021.
02.014] [PMID]
7. Dudek KA, Dion‐Albert L, Kaufmann FN, Tuck E, Lebel M, Menard C. Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. European Journal of Neuroscience. 2021; 53(1):183-221. [DOI:10.1111/ejn.14547] [PMID] [PMCID]
8. Lima-Ojeda JM, Rupprecht R, Baghai TC. Neurobiology of depression: a neurodevelopmental approach. The World Journal of Biological Psychiatry. 2018; 19(5):349-59. [DOI:10.1080/15622975.2017.1289240] [PMID]
9. Linde K, Rücker G, Sigterman K, Jamil S, Meissner K, Schneider A, et al. Comparative effectiveness of psychological treatments for depressive disorders in primary care: network meta-analysis. BMC Family Practice. 2015; 16(1):1-14. [DOI:10.1186/s12875-015-0314-x] [PMID] [PMCID]
10. Young N. Non-pharmacological treatments for patients with depression. Nursing Standard. 2013; 28(7):43-51. [DOI:10.
7748/ns2013.10.28.7.43.e7577]
11. Zamani M, Radahmadi M, Reisi P. Therapeutic effects of exercise, escitalopram and exercise-accompanied escitalopram on brain functions in rats with depression. Physiology and Pharmacology. 2022; 26(2):188-99. [DOI:10.52547/phypha.26.2.7]
12. Joodaki M, Radahmadi M, Alaei H. Comparing the Therapeutic Effects of Crocin, Escitalopram and Co-Administration of Escitalopram and Crocin on Learning and Memory in Rats with Stress-Induced Depression. The Malaysian Journal of Medical Sciences. 2021; 28(4):50. [DOI:10.21315/mjms2021.28.4.6] [PMID] [PMCID]
13. Fiori LM, Turecki G. Broadening our horizons: gene expression profiling to help better understand the neurobiology of suicide and depression. Neurobiology of Disease. 2012; 45(1):14-22. [DOI:10.1016/j.nbd.2010.
11.004] [PMID]
14. Palazidou E. The neurobiology of depression. British Medical Bulletin. 2012; 101(1):127-45. [DOI:10.1093/bmb/
lds004] [PMID]
15. Zalsman G, Huang Y-y, Oquendo MA, Burke AK, Hu X-z, Brent DA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. American Journal of Psychiatry. 2006; 163(9):1588-93. [DOI:10.1176/ajp.2006.163.9.1588] [PMID]
16. Skoog I, Waern M, Duberstein P, Blennow K, Zetterberg H, Börjesson-Hanson A, et al. A 9-year prospective population-based study on the association between the APOE* E4 allele and late-life depression in Sweden. Biological Psychiatry. 2015; 78(10):730-6. [DOI:10.1016/j.biopsych.2015.01.006] [PMID]
17. Kang H-J, Park Y, Yoo K-H, Kim K-T, Kim E-S, Kim J-W, et al. Sex differences in the genetic architecture of depression. Scientific Reports. 2020; 10(1):1-12. [DOI:10.1038/s41598-020-66672-9] [PMID]
18. Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. Journal of Neuroscience. 2008; 28(36):9055-65. [DOI:10.1523/
JNEUROSCI.1424-08.2008] [PMID] [PMCID]
19. Verduijn J, Milaneschi Y, Schoevers RA, van Hemert AM, Beekman AT, Penninx BW. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Translational Psychiatry. 2015; 5(9):e649-e. [DOI:10.1038/tp.2015.137] [PMID] [PMCID]
20. Agelink MW, Klimke A, Cordes J, Sanner D, Kavuk I, Malessa R, et al. A functional-structural model to understand cardiac autonomic nervous system (ANS) dysregulation in affective illness and to elucidate the ANS effects of antidepressive treatment. European Journal of Medical Research. 2004; 9(1):37-50. [PMID]
21. Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B12 deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. American Journal of Psychiatry. 2000; 157(5):715-21. [DOI:10.1176/
appi.ajp.157.5.715] [PMID]
22. Leonard BE. The HPA and immune axes in stress: the involvement of the serotonergic system. European Psychiatry. 2005; 20:S302-S6. [DOI:10.1016/s0924-9338
(05)80180-4] [PMID]
23. Gilman SE, Trinh N-H, Smoller JW, Fava M, Murphy JM, Breslau J. Psychosocial stressors and the prognosis of major depression: a test of Axis IV. Psychological Medicine. 2013; 43(2):303-16. [DOI:10.1017/S0033291712001080] [PMID]
24. Cohen S, Murphy ML, Prather AA. Ten surprising facts about stressful life events and disease risk. Annual review of psychology. 2019; 70:577. [DOI:10.1146/annurev-psych-010418-102857] [PMID]
25. Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nature Reviews Neuroscience. 2016; 17(8):497. [DOI:10.1038/nrn.2016.69] [PMID] [PMCID]
26. Li J, Chen J, Ma N, Yan D, Wang Y, Zhao X, et al. Effects of corticosterone on the expression of mature brain-derived neurotrophic factor (mBDNF) and proBDNF in the hippocampal dentate gyrus. Behavioural Brain Research. 2019; 365:150-6. [DOI:10.1016/j.bbr.2019.03.010] [PMID]
27. Sgoifo A, Carnevali L, Pico Alfonso MdlA, Amore M. Autonomic dysfunction and heart rate variability in depression. Stress. 2015; 18(3):343-52. [DOI:10.3109/102
53890.2015.1045868] [PMID]
28. Murray G. Diurnal mood variation in depression: a signal of disturbed circadian function? Journal of affective Disorders. 2007; 102(1-3):47-53. [DOI:10.1016/j.jad.2006.12.001] [PMID]
29. Ma L, Xu Y, Wang G, Li R. What do we know about sex differences in depression: a review of animal models and potential mechanisms. Progress in Neuro-Psycho-pharmacology and Biological Psychiatry. 2019; 89:48-56. [DOI:10.1016/j.pnpbp.2018.08.026] [PMID]
30. Van Wingen GA, Ossewaarde L, Bäckström T, Hermans EJ, Fernández G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011; 191:38-45. [DOI:10.1016/j.neuroscience.2011.04.042] [PMID]
31. Long Z, Duan X, Wang Y, Liu F, Zeng L, Zhao J-p, et al. Disrupted structural connectivity network in treatment-naive depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015; 56:18-26. [DOI:10.1016/j.
pnpbp.2014.07.007] [PMID]
32. Cullen KR, Klimes-Dougan B, Muetzel R, Mueller BA, Camchong J, Houri A, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010; 49(2):173-83. e1. [DOI:10.1097/00004583-201002000-00011] [PMID] [PMCID]
33. Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Developmental neurobiology. 2010; 70(5):289-97. [DOI:10.1002/dneu.20758] [PMID]
34. Castrén E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 2013; 70(9):983-9. [DOI:10.
1001/jamapsychiatry.2013.1] [PMID]
35. Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Khan S, et al. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002; 51(4):273-9. [DOI:10.1016/s0006-3223(01)01336-1] [PMID]
36. Avery JA, Drevets WC, Moseman SE, Bodurka J, Barcalow JC, Simmons WK. Major depressive disorder is associated with abnormal interoceptive activity and functional connectivity in the insula. Biological Psychiatry. 2014; 76(3):258-66. [DOI:
10.1016/j.biopsych.2013.11.027] [PMID]
37. Boas GRV, de Lacerda RB, Paes MM, Gubert P, da Cruz Almeida WL, Rescia VC, et al. Molecular aspects of depression: a review from neurobiology to treatment. European Journal of Pharmacology. 2019; 851:99-121. [DOI:10.1016/j.ejphar.2019.02.024] [PMID]
38. Palmer SM, Crewther SG, Carey LM. A meta-analysis of changes in brain activity in clinical depression. Frontiers in Human Neuroscience. 2015; 8:1045. [DOI:10.3389/
fnhum.2014.01045]
39. Kern N, Sheldrick AJ, Schmidt FM, Minkwitz J. Neurobiology of depression and novel antidepressant drug targets. Current Pharmaceutical Design. 2012; 18(36):5791-801. [DOI:10.2174/138161212803523581] [PMID]
40. Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: Psychoradiological evidence. CNS Neuroscience & Therapeutics. 2018; 24(11):
994-1003. [DOI:10.1111/cns.12835] [PMID]
41. Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in Neurobiology. 2012; 98(1):99-143. [DOI:10.1016/j.pneurobio.2012.05.009] [PMID]
42. Gerritsen L, Comijs HC, van der Graaf Y, Knoops AJ, Penninx BW, Geerlings MI. Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—the SMART Medea study. Biological psychiatry. 2011; 70(4):373-80. [DOI:10.1016/j.biopsych.
2011.01.029] [PMID]
43. Frodl T, Meisenzahl EM, Zetzsche T, Born C, Groll C, Jäger M, et al. Hippocampal changes in patients with a first episode of major depression. American Journal of Psychiatry. 2002; 159(7):1112-8. [DOI:10.1176/appi.ajp.
159.7.1112] [PMID]
44. Jain N, Steffens DC. Neurobiology and risk factors of late-life depression. Understanding Depression. Springer; 2018.
45. Thakker-Varia S, Alder J. Neuropeptides in depression: role of VGF. Behavioural Brain Research. 2009; 197(2):262-78. [DOI:10.1016/j.bbr.2008.10.006] [PMID] [PMCID]
46. Fossati P, Radtchenko A, Boyer P. Neuroplasticity: from MRI to depressive symptoms. European Neuropsycho-pharmacology. 2004; 14:S503-S10. [DOI:10.1016/j.euro
neuro.2004.09.001] [PMID]
47. Gold PW, Machado-Vieira R, Pavlatou MG. Clinical and biochemical manifestations of depression: relation to the neurobiology of stress. Neural plasticity. 2015; 2015:581976. [DOI:10.1155/2015/581976] [PMID] [PMCID]
48. Bowley MP, Drevets WC, Öngür D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biological Psychiatry. 2002; 52(5):404-12. [DOI:10.1016/
s0006-3223(02)01404-x] [PMID]
49. Huang P, Gao T, Dong Z, Zhou C, Lai Y, Pan T, et al. Neural circuitry among connecting the hippocampus, prefrontal cortex and basolateral amygdala in a mouse depression model: associations correlations between BDNF levels and BOLD–fMRI signals. Brain Research Bulletin. 2018; 142:107-15. [DOI:10.1016/j.brainresbull.2018.06.
019] [PMID]
50. Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annual review of medicine. 1998; 49(1):341-61. [DOI:10.1146/annurev.med.49.1.341] [PMID]
51. Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsycho-pharmacology. 2006; 31(4):814-24. [DOI:10.1038/sj.npp.
1300897] [PMID]
52. Bhagya V, Srikumar B, Raju T, Rao BS. Chronic escitalopram treatment restores spatial learning, monoamine levels, and hippocampal long-term potentiation in an animal model of depression. Psychopharmacology. 2011; 214(2):477-94. [DOI:10.1007/s00213-010-2054-x] [PMID]
53. Nugent AC, Davis RM, Zarate Jr CA, Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry Research: Neuroimaging. 2013; 213(3):179-85. [DOI:10.
1016/j.pscychresns.2013.05.004] [PMID]
54. Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, et al. The insular cortex and the neuroanatomy of major depression. Journal of Affective Disorders. 2011; 133(1-2):120-7. [DOI:10.1016/j.jad.
2011.04.004] [PMID] [PMCID]
55. Willner P, Scheel-Krüger J, Belzung C. The neurobiology of depression and antidepressant action. Neuroscience & Biobehavioral Reviews. 2013; 37(10):2331-71. [DOI:
10.1016/j.neubiorev.2012.12.007] [PMID]
56. Dean J, Keshavan M. The neurobiology of depression: An integrated view. Asian journal of psychiatry. 2017; 27:101-11. [DOI:10.1016/j.ajp.2017.01.025] [PMID]
57. Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Translational psychiatry. 2012; 2(2):e79-e. [DOI:10.1038/tp.2012.8] [PMID] [PMCID]
58. Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010; 34(5):766-76. [DOI:10.1016/j.pnpbp.
2009.09.006] [PMID]
59. Pitsillou E, Bresnehan SM, Kagarakis EA, Wijoyo SJ, Liang J, Hung A, et al. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Molecular Biology Reports. 2020; 47(1):753-70. [DOI:10.1007/s11033-019-05129-3] [PMID]
60. Ferrari F, Villa R. The neurobiology of depression: an integrated overview from biological theories to clinical evidence. Molecular Neurobiology. 2017; 54(7):4847-65. [DOI:10.1007/s12035-016-0032-y] [PMID]
61. Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. The British Journal of Psychiatry. 2001; 178(5):399-405. [DOI:10.1192/bjp.178.5.399] [PMID]
62. Meneses A, Liy-Salmeron G. Serotonin and emotion, learning and memory. Reviews in the Neurosciences. 2012; 23(5-6):543-53. [DOI:10.1515/revneuro-2012-0060] [PMID]
63. Chopra K, Kumar B, Kuhad A. Pathobiological targets of depression. Expert Opinion on Therapeutic Targets. 2011; 15(4):379-400. [DOI:10.1517/14728222.2011.553603] [PMID]
64. Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neuroscience & Biobehavioral Reviews. 2012; 36(9):2085-117. [DOI:10.1016/j.neubiorev.2012.07.001] [PMID] [PMID]
65. Mann J, Currier D. Stress, genetics and epigenetic effects on the neurobiology of suicidal behavior and depression. European Psychiatry. 2010; 25(5):268-71. [DOI:10.1016/j.
eurpsy.2010.01.009] [PMID] [PMCID]
66. Chen MJ. The neurobiology of depression and physical exercise. InRoutledge handbook of physical activity and mental health: Routledge; 2013.
67. Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Research Reviews. 2009; 61(2):105-23. [DOI:10.1016/j.
brainresrev.2009.05.005] [PMID]
68. Cathomas F, Murrough JW, Nestler EJ, Han M-H, Russo SJ. Neurobiology of resilience: interface between mind and body. Biological Psychiatry. 2019; 86(6):410-20. [DOI:10.
1016/j.biopsych.2019.04.011] [PMID]
69. Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013; 493(7433):532-6. [DOI:10.1038/nature11713] [PMID] [PMCID]
70. Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Therapeutic Advances in Chronic Disease. 2015; 6(3):97-114. [DOI:10.1177/2040
622315579059] [PMID] [PMCID]
71. Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neuroscience & Biobehavioral Reviews. 2011; 35(3):537-55. [DOI:10.1016/j.neubiorev.2010.06.006] [PMID] [PMCID]
72. Murrough JW, Abdallah CG, Mathew SJ. Targeting glutamate signalling in depression: progress and prospects. Nature Reviews Drug Discovery. 2017; 16(7):472-86. [DOI:10.1038/nrd.2017.16] [PMID]
73. Duman RS, Shinohara R, Fogaça MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Molecular psychiatry. 2019:1-17. [DOI: 10.1038/s41380-019-0400-x] [PMID] [PMCID]
74. Frisch P, Bilkei-Gorzó A, Rácz I, Zimmer A. Modulation of the CRH system by substance P/NKA in an animal model of depression. Behavioural Brain Research. 2010; 213(1):103-8. [DOI:10.1016/j.bbr.2010.04.044] [PMID]
75. Ranga K, Krishnan R. Clinical experience with substance P receptor (NK1) antagonists in depression. The Journal of Clinical Psychiatry. 2002; 63:25. [PMID]
76. Bondy B, Baghai TC, Minov C, Schüle C, Schwarz MJ, Zwanzger P, et al. Substance P serum levels are increased in major depression: preliminary results. Biological Psychiatry. 2003; 53(6):538-42. [DOI:10.1016/s0006-3223(02)01544-5] [PMID]
77. Keller M, Montgomery S, Ball W, Morrison M, Snavely D, Liu G, et al. Lack of efficacy of the substance p (neurokinin1 receptor) antagonist aprepitant in the treatment of major depressive disorder. Biological Psychiatry. 2006; 59(3):216-23. [DOI:10.1016/j.biopsych.2005.07.013] [PMID]
78. Narasimhan M, Campbell N. A tale of two comorbidities: understanding the neurobiology of depression and pain. Indian Journal of Psychiatry. 2010; 52(2):127. [DOI:10.4103/0019-5545.64586] [PMID]
79. Caraci F, Calabrese F, Molteni R, Bartova L, Dold M, Leggio GM, et al. International :union: of basic and clinical pharmacology CIV: The neurobiology of treatment-resistant depression: From antidepressant classifications to novel pharmacological targets. Pharmacological Reviews. 2018; 70(3):475-504. [DOI:10.1124/pr.117.014977] [PMID]
80. Ittermann T, Völzke H, Baumeister SE, Appel K, Grabe HJ. Diagnosed thyroid disorders are associated with depression and anxiety. Social Psychiatry and Psychiatric Epidemiology. 2015; 50(9):1417-25. [DOI:10.1007/s00127-015-1043-0] [PMID]
81. Berent D, Zboralski K, Orzechowska A, Gałecki P. Thyroid hormones association with depression severity and clinical outcome in patients with major depressive disorder. Molecular Biology Reports. 2014; 41(4):2419-25. [DOI:10.1007/s11033-014-3097-6] [PMID] [PMCID]
82. Yu D, Zhou H, Yang Y, Jiang Y, Wang T, Lv L, et al. The bidirectional effects of hypothyroidism and hyperthyroidism on anxiety-and depression-like behaviors in rats. Hormones and Behavior. 2015; 69:106-15. [DOI:10.1016/j.yhbeh.
2015.01.003] [PMID]
83. Bauer M, Whybrow P. Role of thyroid hormone therapy in depressive disorders. Journal of Endocrinological Investigation. 2021; 44(11):2341-7. [DOI:10.1007/s40618-021-01600-w] [PMID] [PMCID]
84. Moon JH, Han JW, Oh TJ, Choi SH, Lim S, Kim KW, et al. Effect of increased levothyroxine dose on depressive mood in older adults undergoing thyroid hormone replacement therapy. Clinical Endocrinology. 2020; 93(2):196-203. [DOI:10.1111/cen.14189] [PMID]
85. Almeida OP, Alfonso H, Flicker L, Hankey G, Chubb SP, Yeap BB. Thyroid hormones and depression: the Health in Men study. The American Journal of Geriatric Psychiatry. 2011; 19(9):763-70. [DOI:10.1097/JGP.0b013e31820dca
d5] [PMID]
86. Engum A, Bjøro T, Mykletun A, Dahl AA. An association between depression, anxiety and thyroid function–a clinical fact or an artefact? Acta Psychiatrica Scandinavica. 2002; 106(1):27-34. [DOI:10.1034/j.1600-0447.2002.01250.x] [PMID]
87. Lorentzen R, Kjaer JN, Østergaard S, Madsen M. Thyroid hormone treatment in the management of treatment‐resistant unipolar depression: a systematic review and meta‐analysis. Acta Psychiatrica Scandinavica. 2020; 141(4):316-26. [DOI:10.1111/acps.13154] [PMID]
88. Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatric Disease and treatment. 2011; 7:9-13. [DOI:10.2147/NDT.S19619] [PMID] [PMCID]
89. Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. Journal of affective disorders. 2003; 74(1):85-96. [DOI:10.1016/s0165-0327(02)00428-7] [PMID] [PMCID]
90. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006; 63(4):375-82. [DOI:10.1001/archpsyc.
63.4.375] [PMID]
91. Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depression and Anxiety. 2007; 24(7):495-517. [DOI:10.1002/da.20262] [PMID]
92. Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Archives of General Psychiatry. 2008; 65(3):283-9. [DOI:10.1001/archgenpsychiatry.2007.33] [PMID]
93. Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008; 33(2):368-77. [DOI:10.1038/sj.npp.1301408] [PMID]
94. Espiritu RP, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA. Depression in primary hyperparathyroidism: prevalence and benefit of surgery. The Journal of Clinical Endocrinology & Metabolism. 2011; 96(11):E1737-E45. [DOI:10.1210/jc.
2011-1486] [PMID]
95. Nissen C, Holz J, Blechert J, Feige B, Riemann D, Voderholzer U, et al. Learning as a model for neural plasticity in major depression. Biological Psychiatry. 2010; 68(6):544-52. [DOI:10.1016/j.biopsych.2010.05.026] [PMID]
96. Zhao G, Ford ES, Li C, Balluz LS. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. British Journal of Nutrition. 2010; 104(11):1696-702. [DOI:10.1017/S0007114510002588] [PMID]
97. Parker KJ, Kenna HA, Zeitzer JM, Keller J, Blasey CM, Amico JA, et al. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Research. 2010; 178(2):359-62. [DOI:10.1016/j.psychres.2009.09.
017] [PMID]
98. Müller MB, Landgraf R, Keck ME. Vasopressin, major depression, and hypothalamic–pituitary–adrenocortical desensitization. Biological Psychiatry. 2000; 48(4):330-3. [DOI:10.1016/s0006-3223(00)00886-6] [PMID]
99. Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: a preliminary report. Biological Psychiatry. 2006; 60(8):892-5. [DOI:10.1016/j.biopsych.2005.12.010] [PMID]
100. Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, et al. Melatonin in mood disorders. The World Journal of Biological Psychiatry. 2006; 7(3):138-51. [DOI:10.1080/15622970600571822] [PMID]
101. Szymanska A, Rabe-Jablonska J, Karasek M. Diurnal profile of melatonin concentrations in patients with major depression: relationship to the clinical manifestation and antidepressant treatment. Neuroendocrinology Letters. 2001; 22(3):192-8. [PMID]
102. Zou XH, Sun LH, Yang W, Li BJ, Cui RJ. Potential role of insulin on the pathogenesis of depression. Cell Proliferation. 2020; 53(5):e12806. [DOI:10.1111/cpr.
12806] [PMID] [PMCID]
103. Dudek KA, Dion‐Albert L, Kaufmann FN, Tuck E, Lebel M, Menard C. Neurobiology of resilience in depression: immune and vascular insights from human and animal studies. European Journal of Neuroscience. 2021; 53(1):183-221. [DOI:10.1111/ejn.14547] [PMID] [PMCID]
104. Na K-S, Lee KJ, Lee JS, Cho YS, Jung H-Y. Efficacy of adjunctive celecoxib treatment for patients with major depressive disorder: a meta-analysis. Progress in Neuro-psychopharmacology and Biological Psychiatry. 2014; 48:79-85. [DOI:10.1016/j.pnpbp.2013.09.006] [PMID]
105. Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological psychiatry. 2010; 68(8):748-54. [DOI:10.1016/
j.biopsych.2010.06.010] [PMID] [PMCID]
106. Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biological Psychiatry. 2010; 67(5):446-57. [DOI:10.1016/j.biopsych.2009.09.033] [PMID]
107. Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB Signaling in the Neurobiology of Depression. Current Pharmaceutical Design. 2017; 23(21):3154-63. [DOI:10.2174/1381612823666170111141915] [PMID]
108. Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. Journal of Neuroscience. 2002; 22(8):3251-61. [DOI:10.1523/JNEUROSCI.22-08-03251.2002] [PMID] [PMCID]
109. Jaggar M, Fanibunda SE, Ghosh S, Duman RS, Vaidya VA. The neurotrophic hypothesis of depression revisited: new insights and therapeutic implications. Neurobiology of Depression: Elsevier; 2019. [DOI:10.1016/B978-0-12-813333-0.00006-8]
110. Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, et al. Reward processing in depression: a conceptual and meta-analytic review across fMRI and EEG studies. American Journal of Psychiatry. 2018; 175(11):1111-20. [DOI:10.1176/appi.ajp.2018.
17101124] [PMID] [PMCID]
111. Satterthwaite TD, Kable JW, Vandekar L, Katchmar N, Bassett DS, Baldassano CF, et al. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015; 40(9):2258-68. [DOI:10.1038/npp.2015.75] [PMID] [PMCID]
112. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007; 64(3):327-37. [DOI:10.1001/archpsyc.
64.3.327] [PMID]
113. Korte SM, Prins J, Krajnc AM, Hendriksen H, Oosting RS, Westphal KG, et al. The many different faces of major depression: it is time for personalized medicine. European journal of Pharmacology. 2015; 753:88-104. [DOI:10.
1016/j.ejphar.2014.11.045] [PMID]
114. Cytokines and Depression: How Your Immune System Causes Depression. Ronald S Smith. Kindle Edition;1997.
115. Cohen-Woods S, Harkess KN. Gene-environment interactions, stress, and depression. 2016.
116. Liu H, Chaudhury D. Understanding mood disorders using electrophysiology and circuit breaking. decoding neural circuit structure and function: Springer; 2017.
117. Maletic V, Robinson M, Oakes T, Iyengar S, Ball S, Russell J. Neurobiology of depression: an integrated view of key findings. International Journal of Clinical Practice. 2007; 61(12):2030-40. [DOI:10.1111/j.1742-1241.2007.01602.x] [PMID] [PMCID]
Article Type: Review Article |
Subject:
Depression
Received: 2022/09/13 | Accepted: 2023/02/14 | Published: 2023/03/19
Received: 2022/09/13 | Accepted: 2023/02/14 | Published: 2023/03/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







